The evolutionary dynamics of untreated HIV and the maintenance of the latent reservoir
Richard Neher
Biozentrum, University of Basel
slides at neherlab.org/201703_UZurich.html
Evolution of HIV
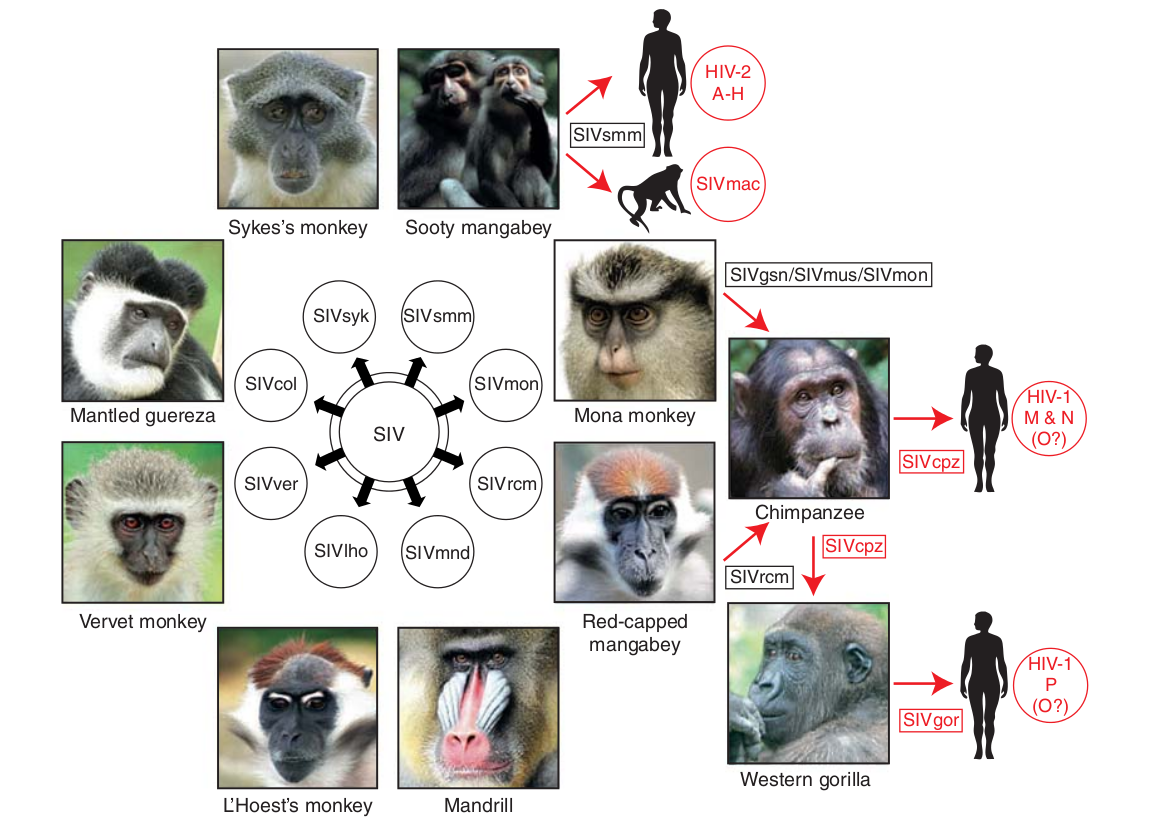
- Chimp → human transmission ~1900 gave rise to HIV-1 group M
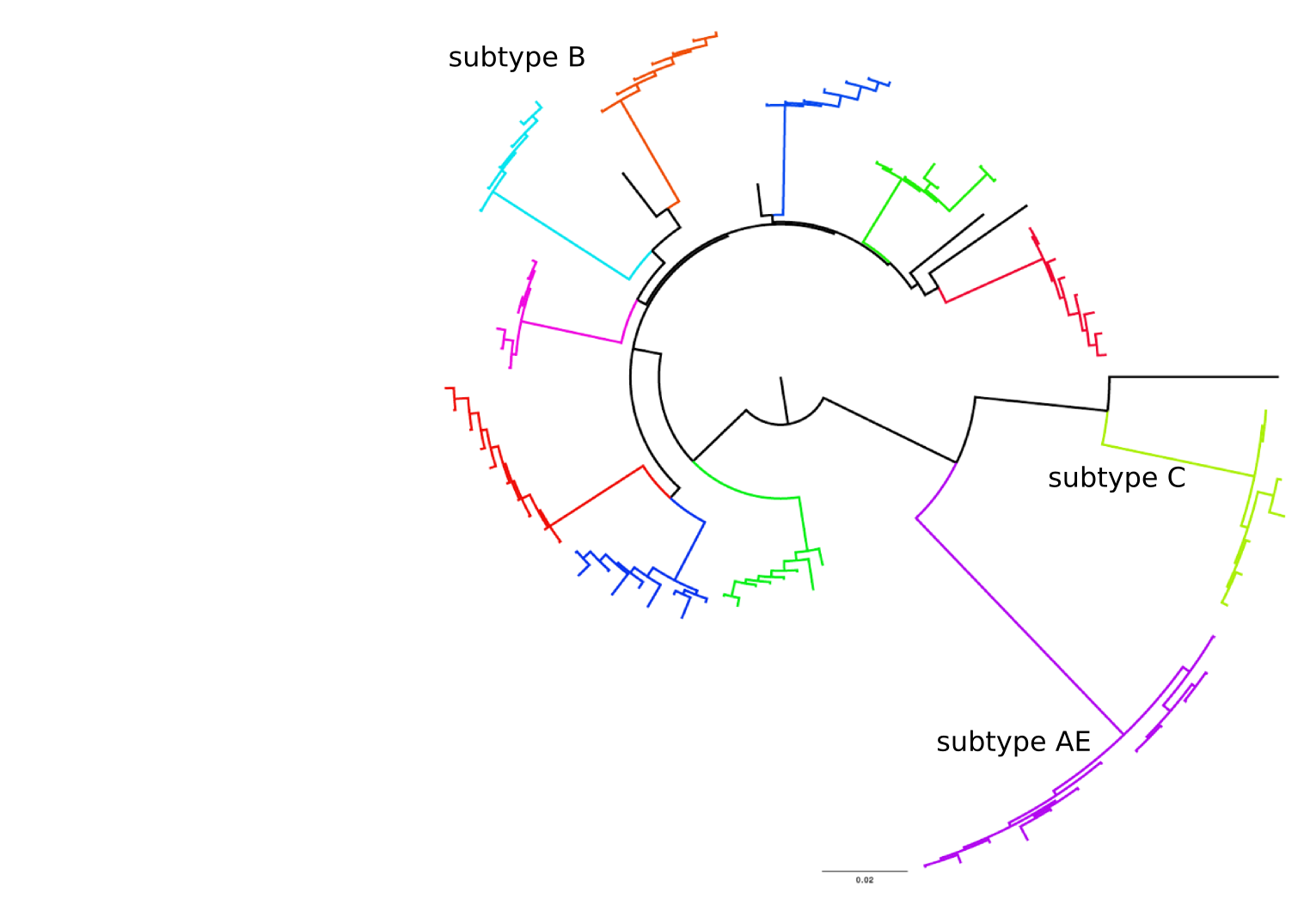
- Diversified into subtypes that are ~20% different
- evolves at a rate of about 0.1% per year
HIV infection
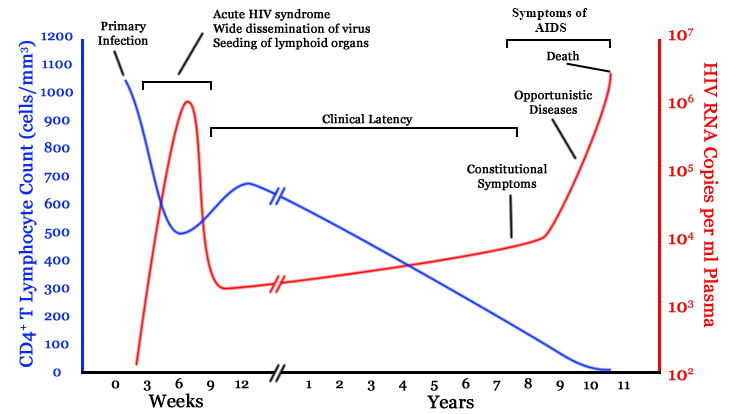 chronic infection:
chronic infection:
- $10^8$ cells are infected every day
- the virus repeatedly escapes immune recognition
- integrates into T-cell as
latent provirus
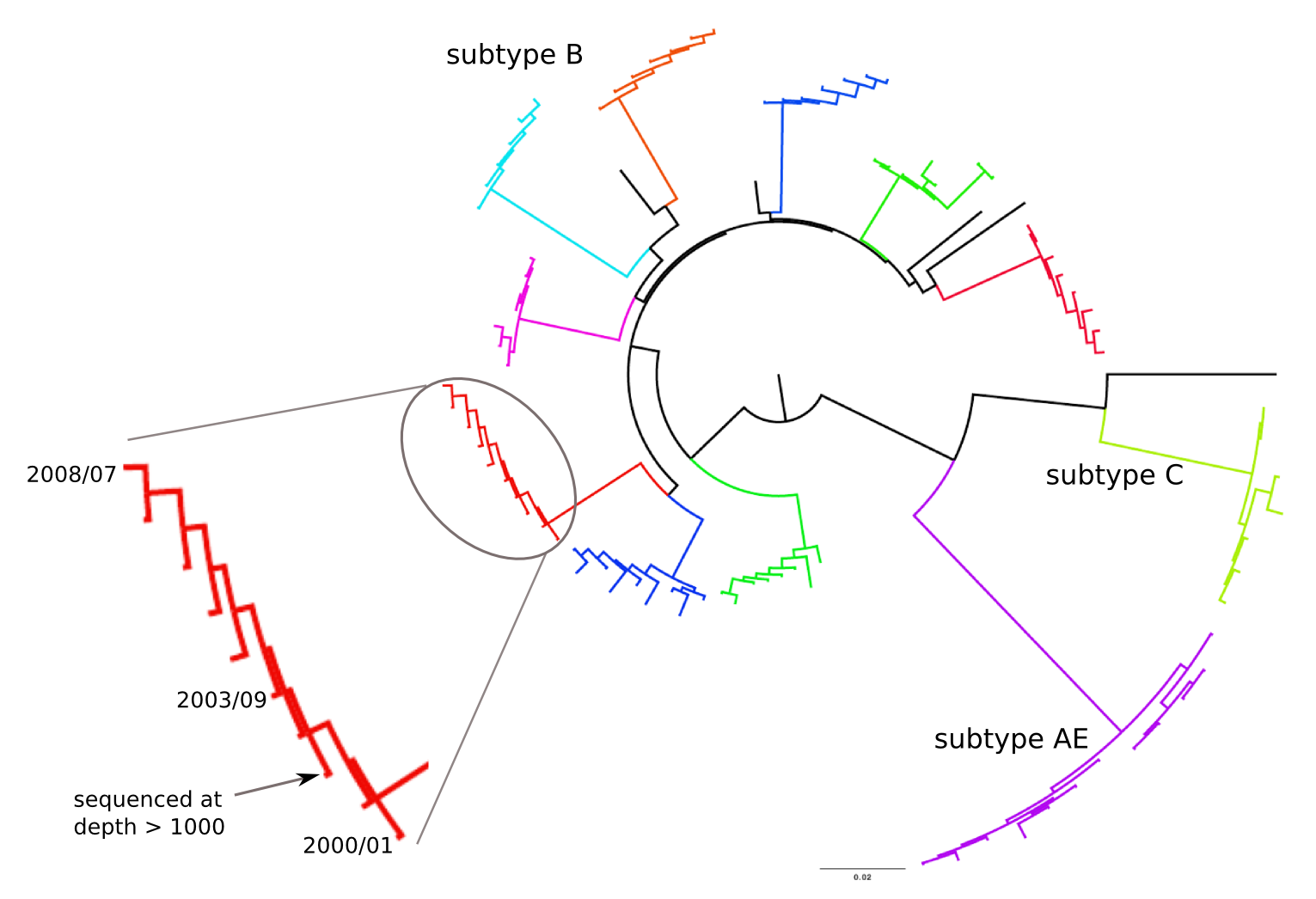
HIV-1 sequencing before and after therapy
 Zanini et al, eLife, 2015;
Brodin et al, eLife, 2016.
Collaboration with the group of Jan Albert
Zanini et al, eLife, 2015;
Brodin et al, eLife, 2016.
Collaboration with the group of Jan Albert
Deep sequencing of plasma RNA
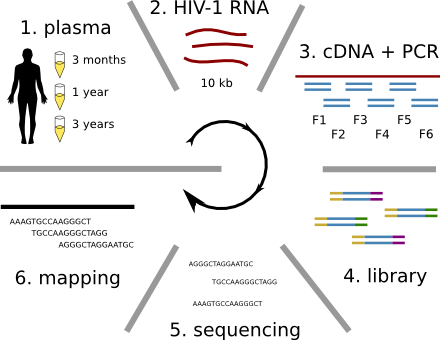
Accuracy of minor variant frequencies
Population sequencing to track all mutations above 1%
- diverge at 0.1-1% per year
- almost full genomes coverage in 10 patients
- full data set at hiv.tuebingen.mpg.de
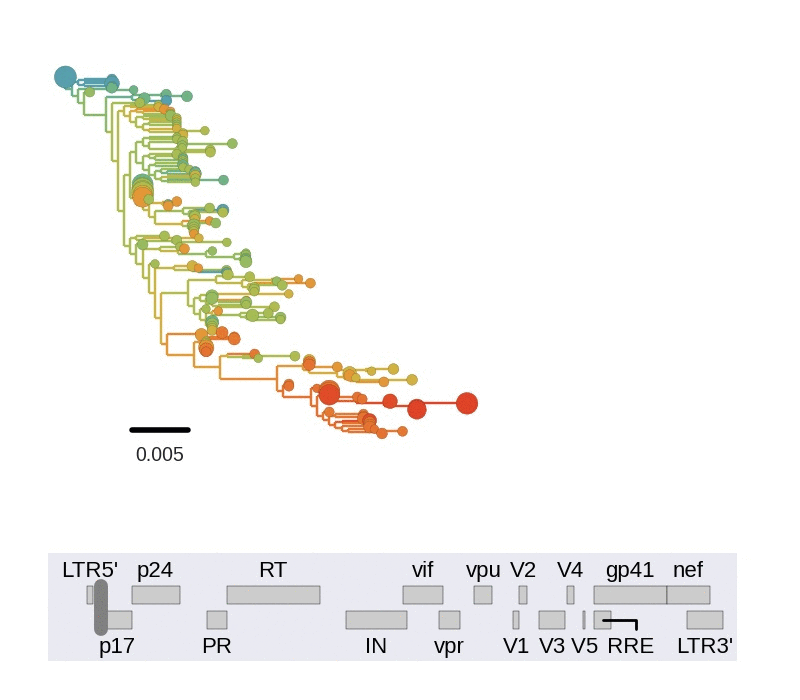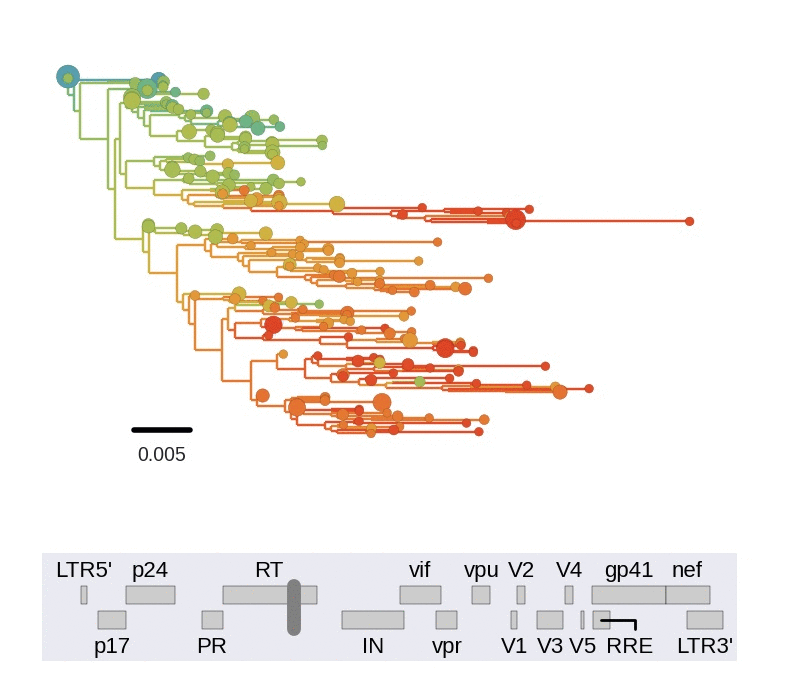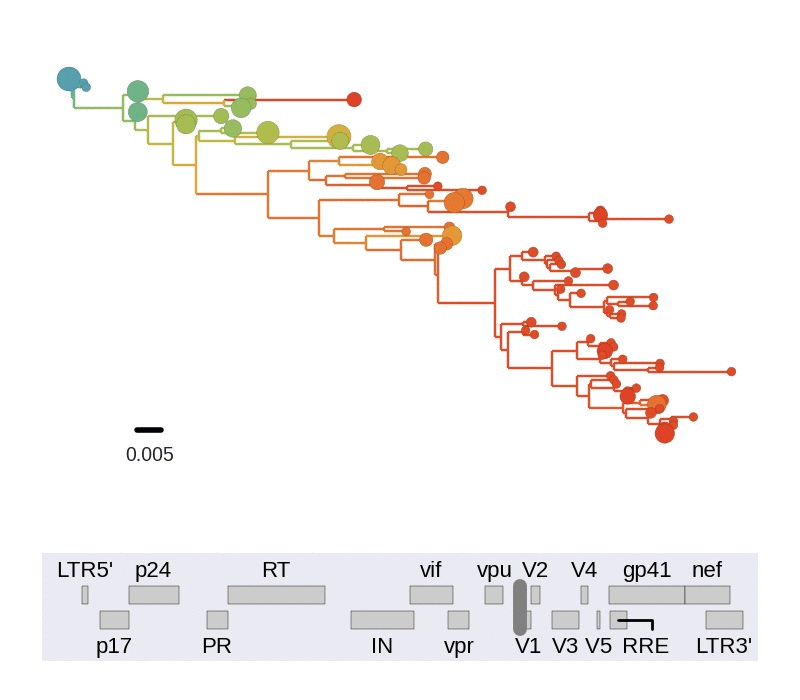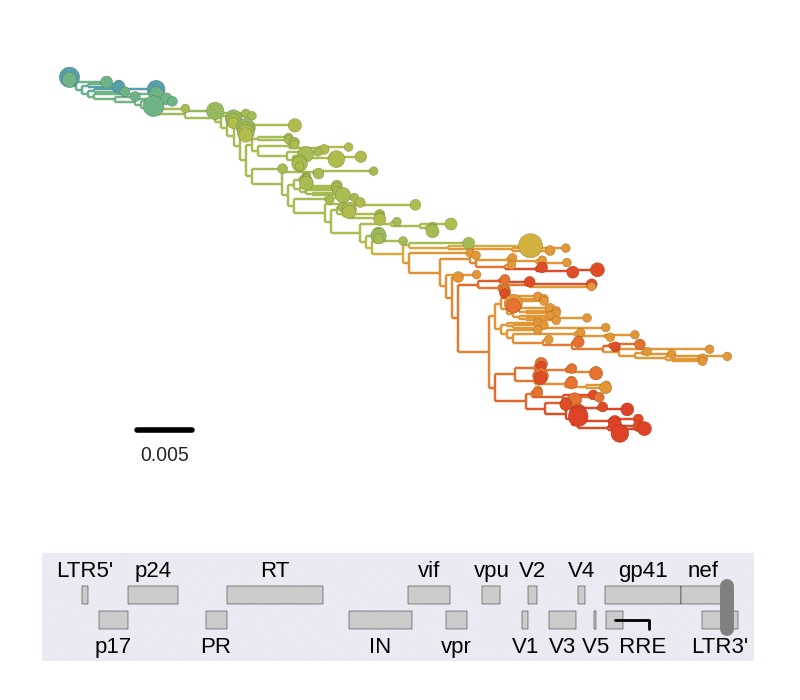
Diversity and mutation rates
- envelope changes fastest, enzymes slowest
- identical rate of synonymous evolution
- diversity saturates where evolution is fast
- synonymous mutations stay at low frequency
Mutation rates and diversity and neutral sites
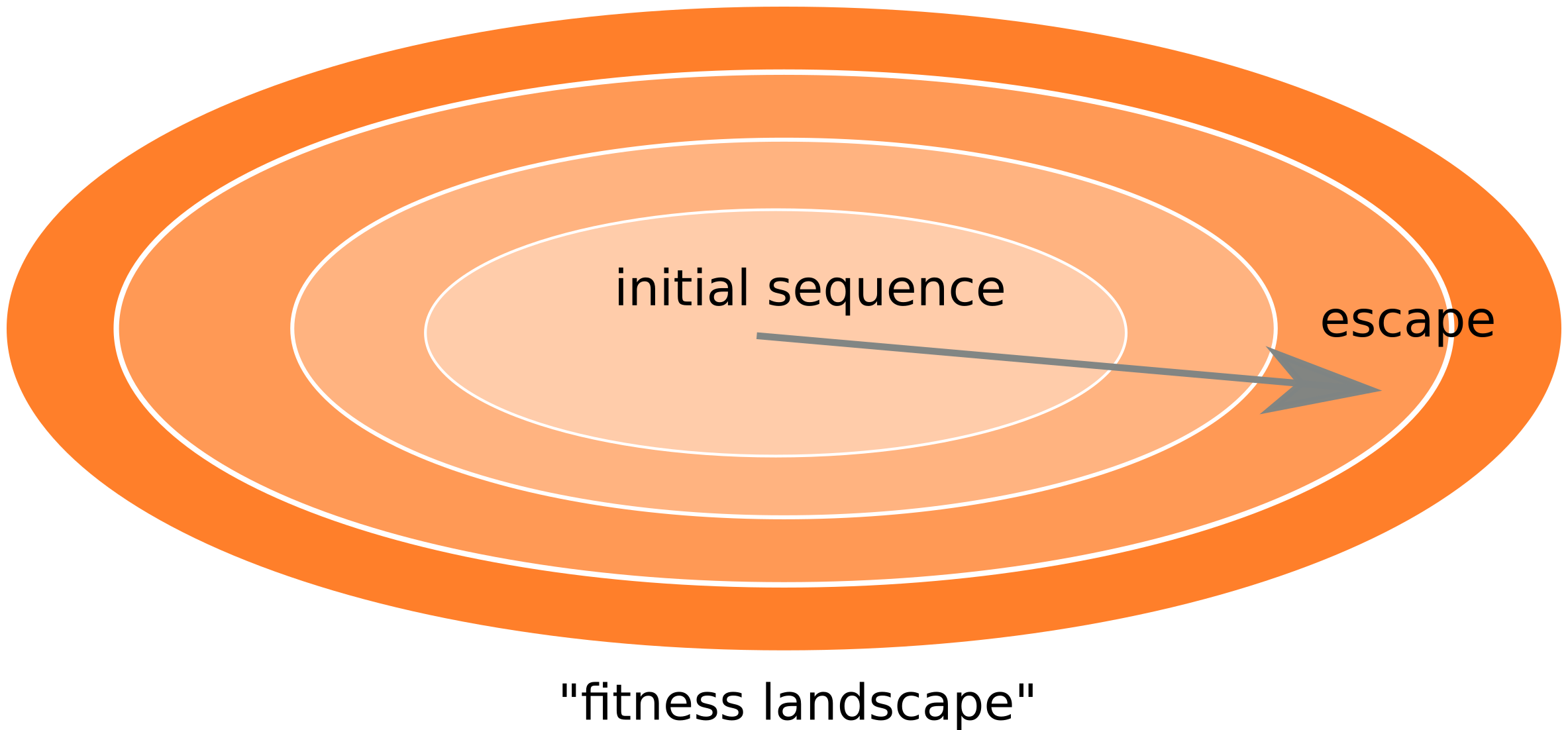
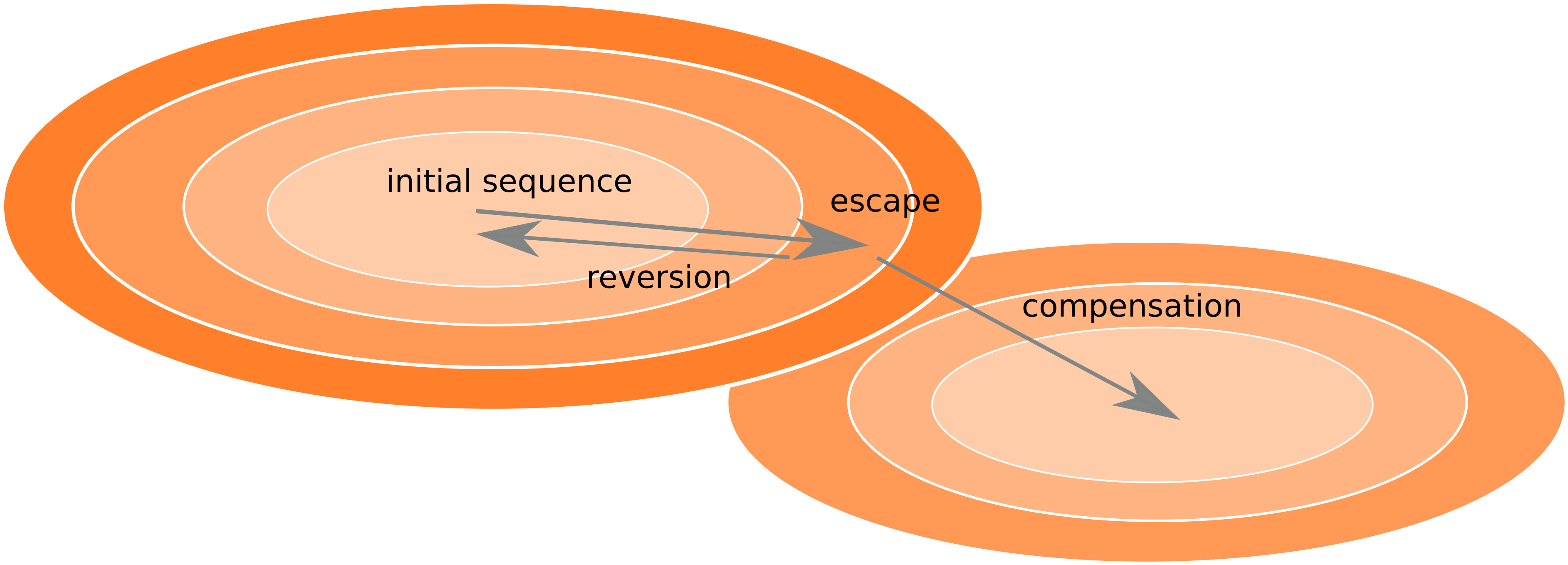
Frequent version of previously beneficial mutations
- HIV escapes immune systems
- most mutations are costly
- humans selects for different mutations
- compensation or reversion?
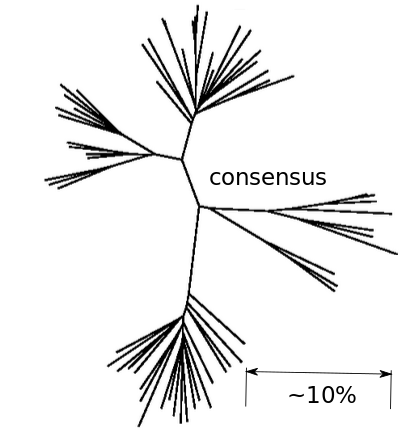
Inference of fitness costs
- mutation away from preferred state with rate $\mu$
- selection against non-preferred state with strength $s$
- variant frequency dynamics: $\frac{d x}{dt} = \mu -s x $
- equilibrium frequency: $\bar{x} = \mu/s $
- fitness cost: $s = \mu/\bar{x}$
Fitness landscape of HIV-1
Zanini et al, Virus Evolution, 2017Selection on RNA structures and regulatory sites
Zanini et al, Virus Evolution, 2017The distribution of fitness costs
Zanini et al, Virus Evolution, 2017Fitness - diversity correlation
Zanini et al, Virus Evolution, 2017Costly HLA associated positions have high diversity
Zanini et al, Virus Evolution, 2017Does HIV evolve during therapy?
 Brodin et al, eLife, 2016
Brodin et al, eLife, 2016
No evidence of ongoing replication
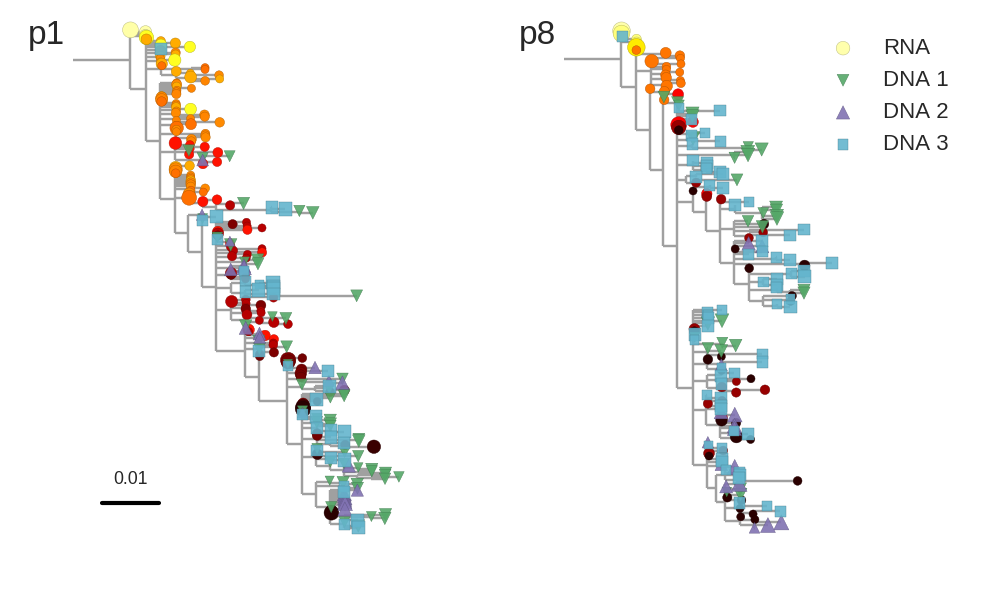
- HIV-1 RNA from plasma before treatment started
- HIV-1 DNA gag-p17 from PBMCs after many years of treatment
No evidence of ongoing replication
Hypermutation
Hypermutation
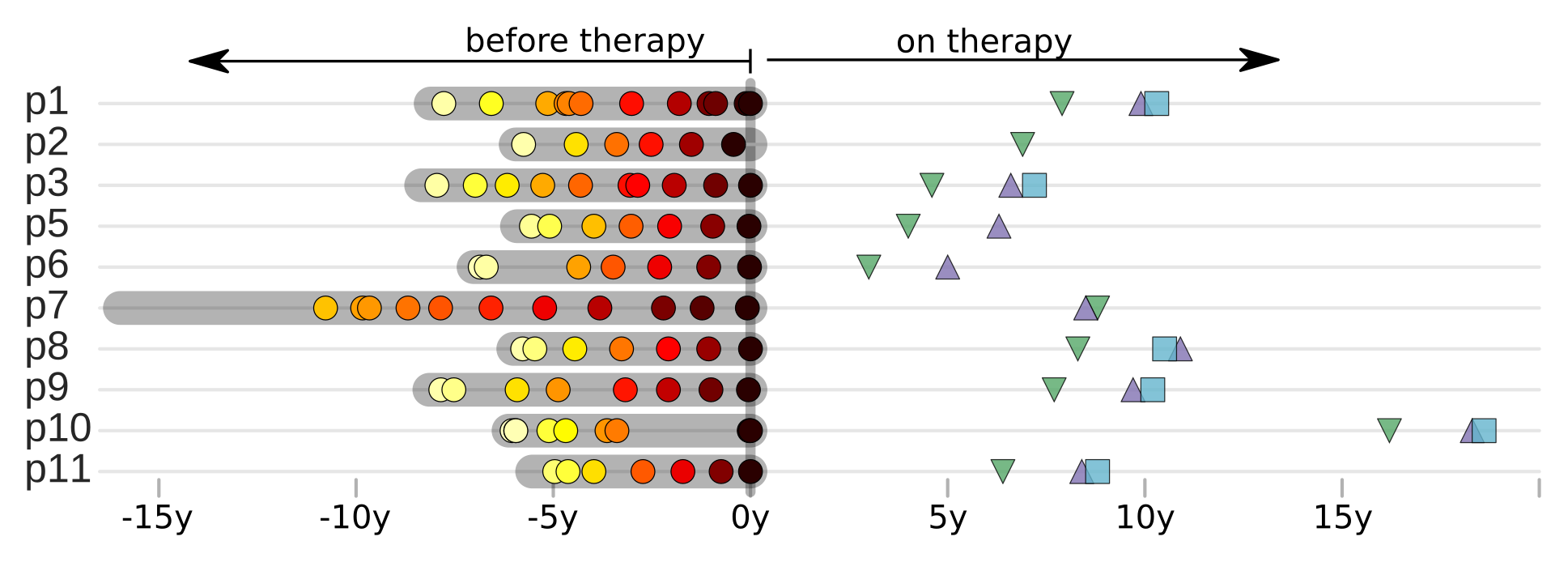
T-cell turnover is fast in untreated infection
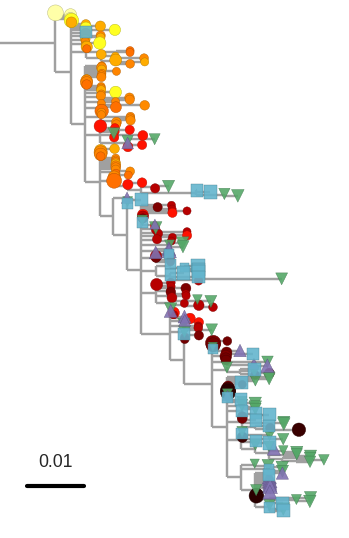
- latent HIV → barcode of a T-cell lineage
- all latent integrated virus derives from late infection
- untreated: T-cell lineages are short lived
- on therapy: T-cell clones live decades
HIV acknowledgments
- Fabio Zanini
- Jan Albert
- Johanna Brodin
- Christa Lanz
- Göran Bratt
- Lina Thebo
- Vadim Puller


the group
