The evolution of untreated HIV and maintenance of the latent reservoir
Richard Neher
Biozentrum, University of Basel
slides at neherlab.org/201809_PEI.html
Evolution of HIV
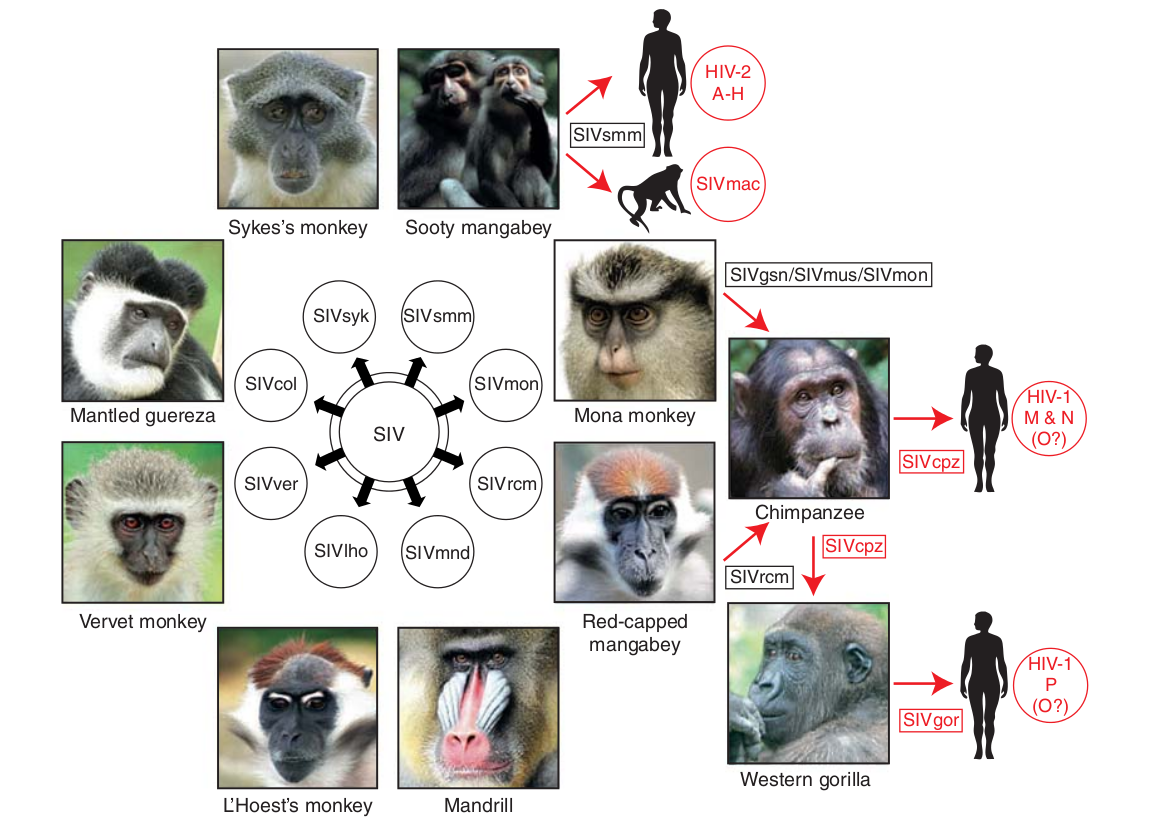
- Chimp → human transmission around 1900 gave rise to HIV-1 group M
- ~100 million infected people since
- subtypes differ at 10-20% of their genome
- HIV-1 evolves ~0.1% per year
HIV infection
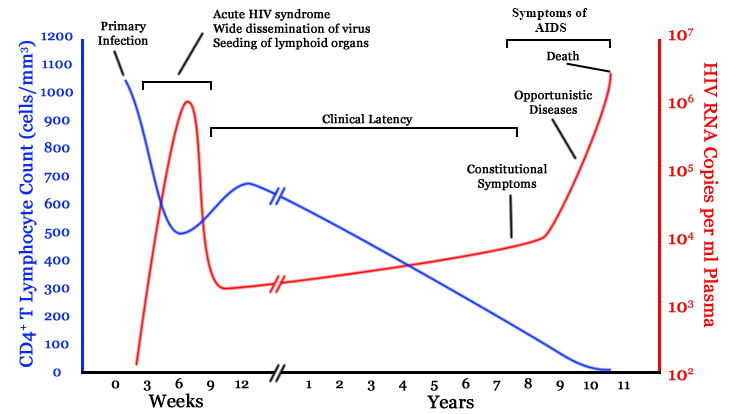
- $10^8$ cells are infected every day
- the virus repeatedly escapes immune recognition
- integrates into T-cells as
latent provirus
HIV-1 evolution within one individual
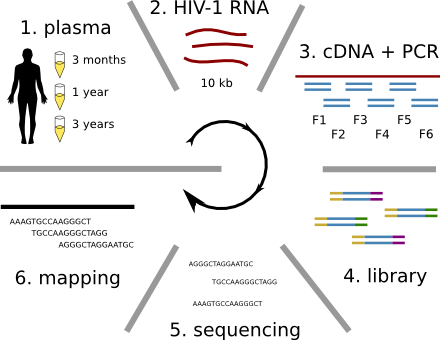

HIV-1 sequencing before and after therapy
 Zanini et al, eLife, 2015;
Brodin et al, eLife, 2016.
Collaboration with the group of Jan Albert
Zanini et al, eLife, 2015;
Brodin et al, eLife, 2016.
Collaboration with the group of Jan Albert
Population sequencing to track all mutations above 1%
Approximately neutral divergence -- silent mutations
In vivo mutation rate estimates
Divergence at increasingly conserved positions
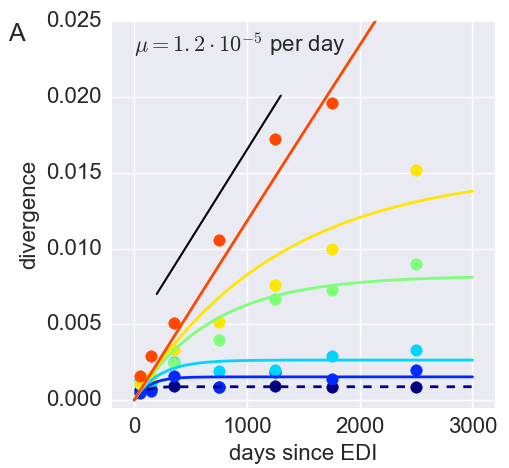
- Six categories from high to low conservation
- mutation away from preferred state with rate $\mu$
- selection against non-preferred state with strength $s$
- variant frequency dynamics: $\frac{d x}{dt} = \mu -s x $
- equilibrium frequency: $\bar{x} = \mu/s $
- fitness cost: $s = \mu/\bar{x}$
- Fit model of minor variation to categories of conservation
- $\Rightarrow$ harmonic average fitness cost in category
Accurate frequency estimates by averaging many samples
- Frequencies of costly mutations decorrelate fast $\frac{d x}{dt} = \mu -s x $
- $\Rightarrow$ average many samples to obtain accurate estimates
Fitness landscape of HIV-1
Zanini et al, Virus Evolution, 2017Selection on RNA structures and regulatory sites
- Blue: all mutations
- Red: only mutations that don't change amino acids
Diversity and hitchhiking
- envelope changes fastest, enzymes lowest
- identical rate of synonymous evolution
- diversity saturates where evolution is fast
- synonymous mutations stay at low frequency
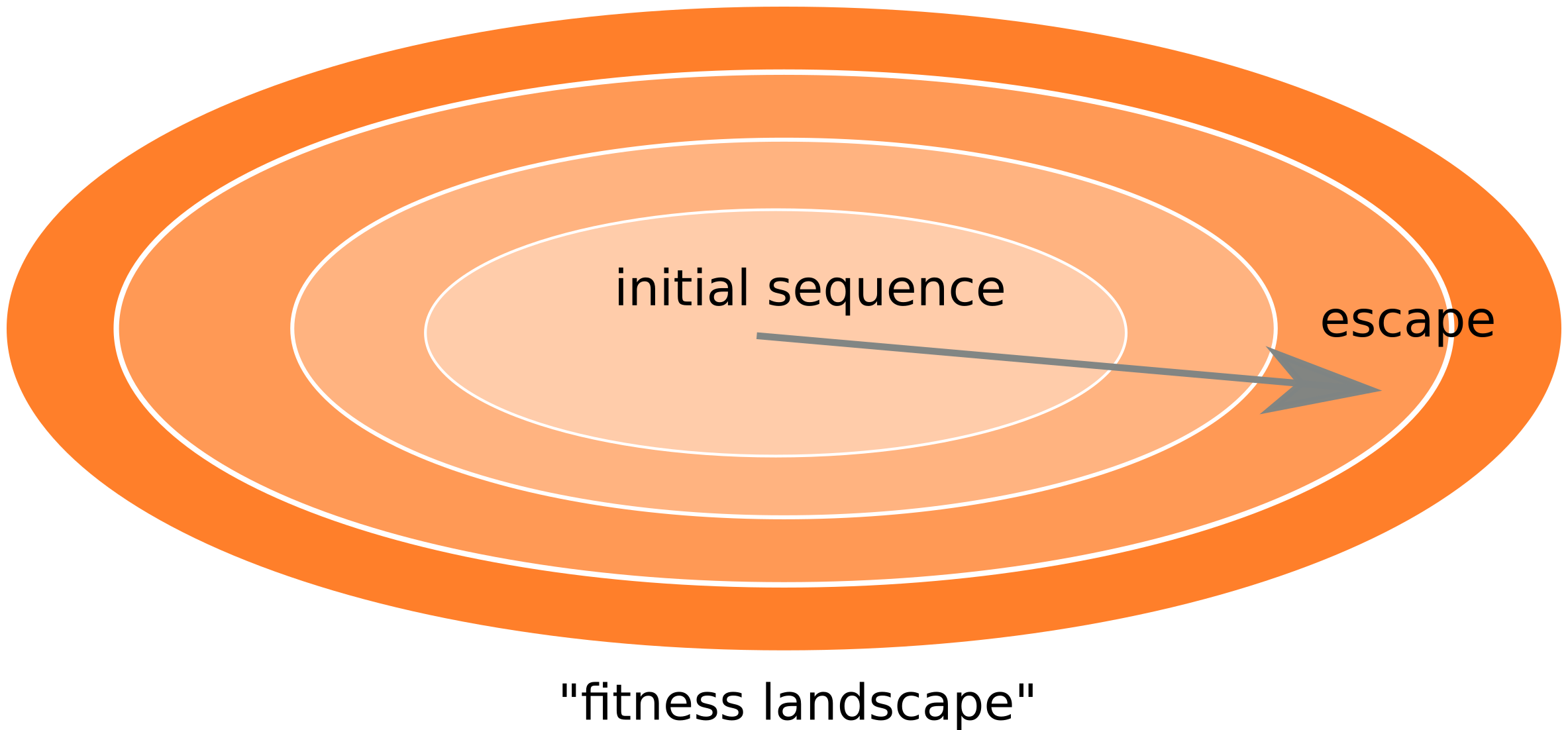
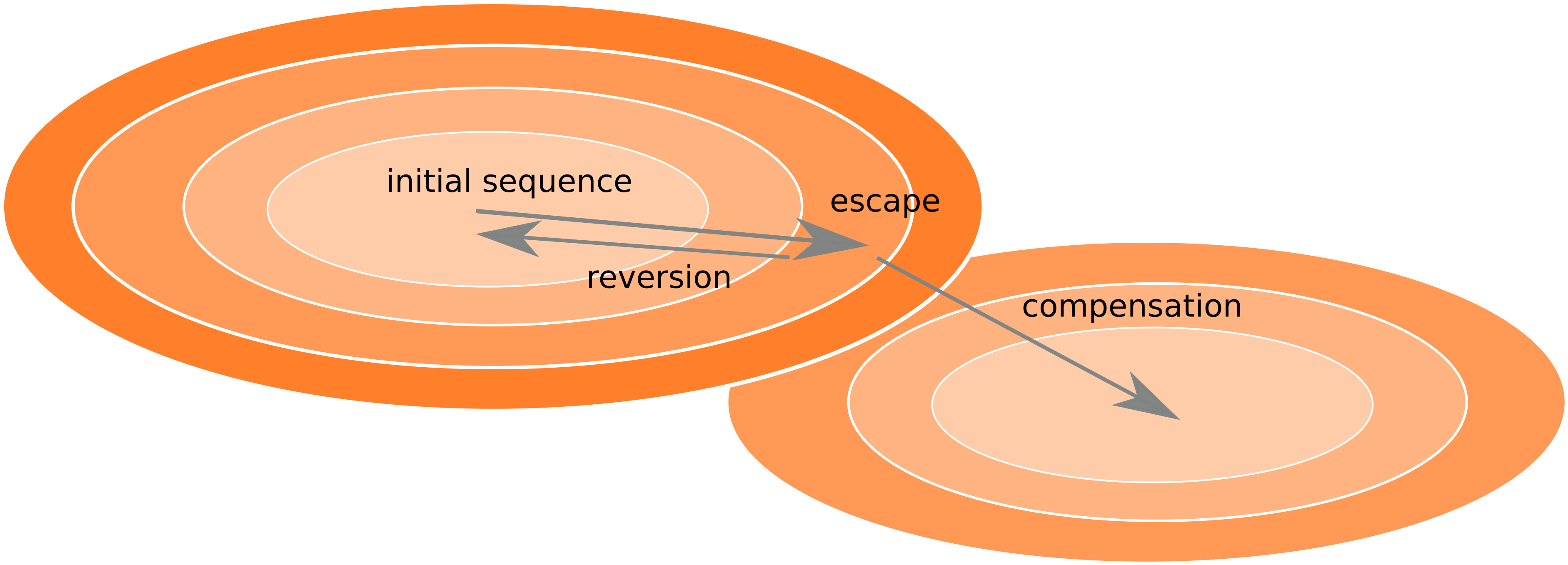
Frequent reversion of previously beneficial mutations
- HIV escapes immune systems
- most mutations are costly
- humans selects for different mutations
- compensation or reversion?
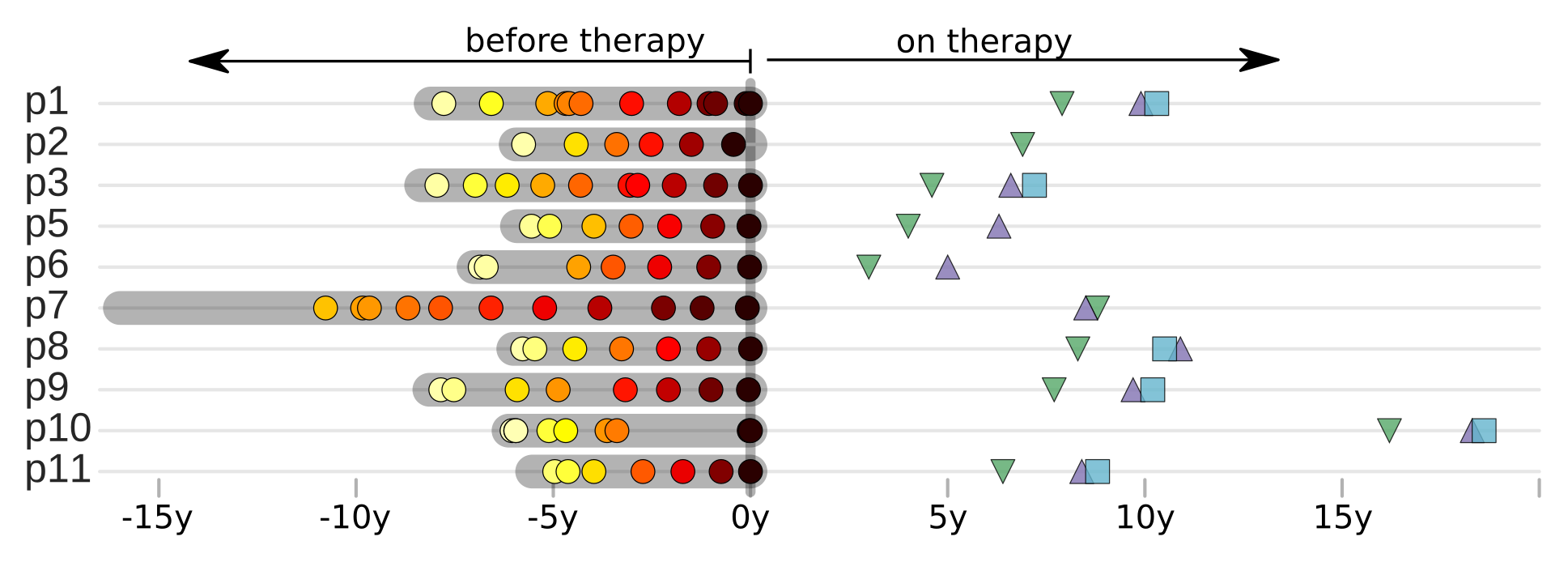
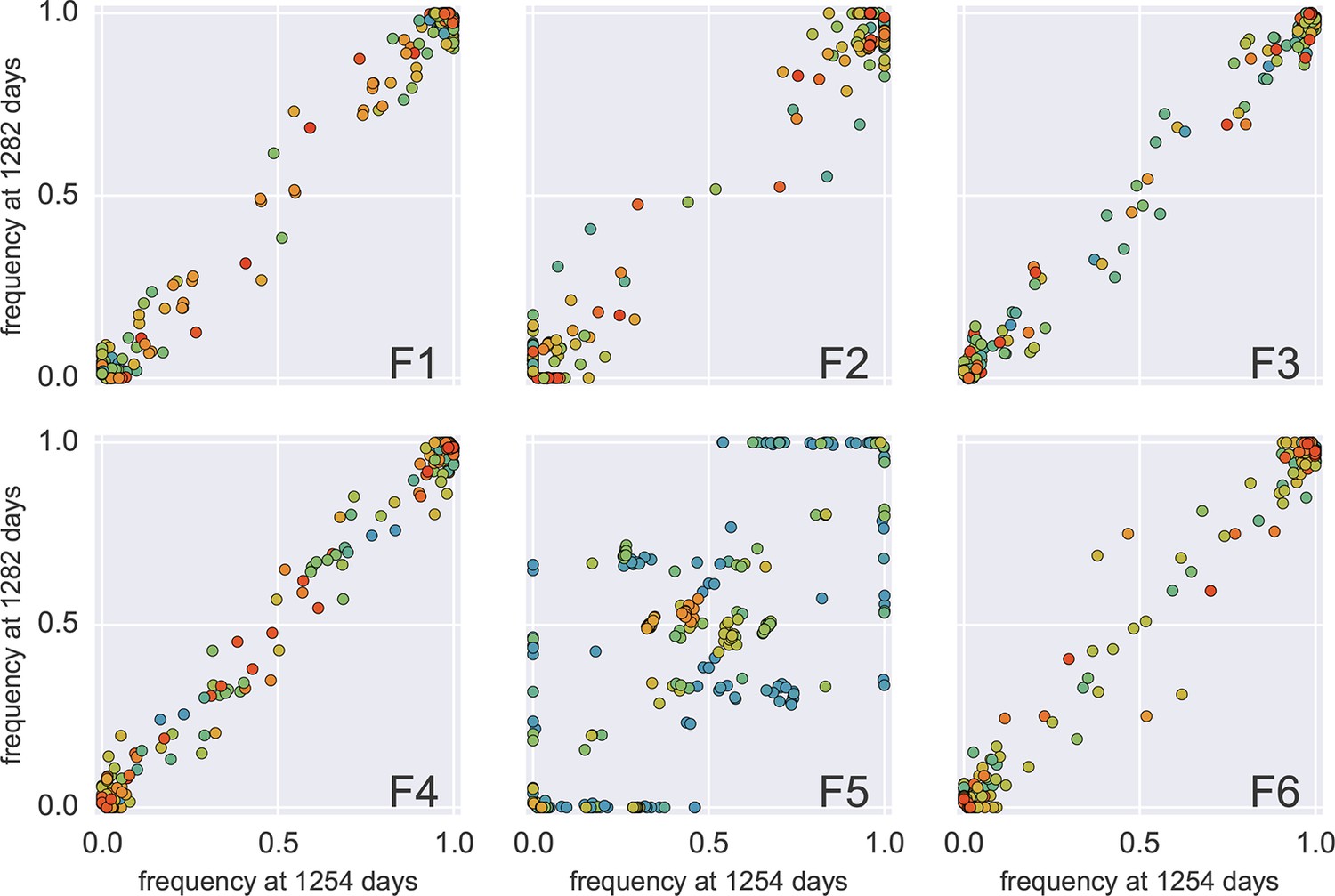
Does HIV evolve during therapy?
 Brodin et al, eLife, 2016
Brodin et al, eLife, 2016
No evidence of ongoing replication
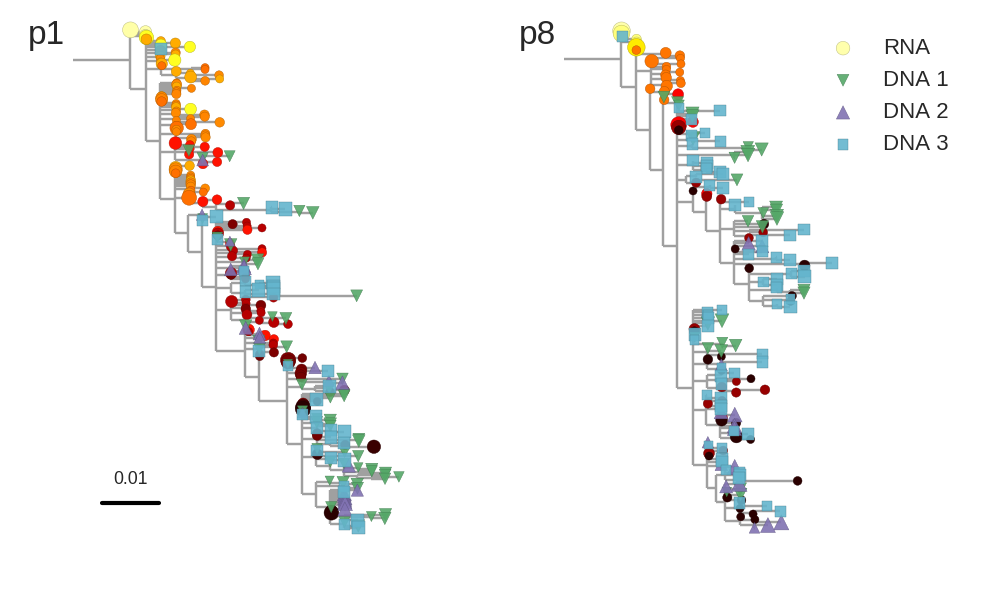
No evidence of ongoing replication
T-cell turnover is fast in untreated infection
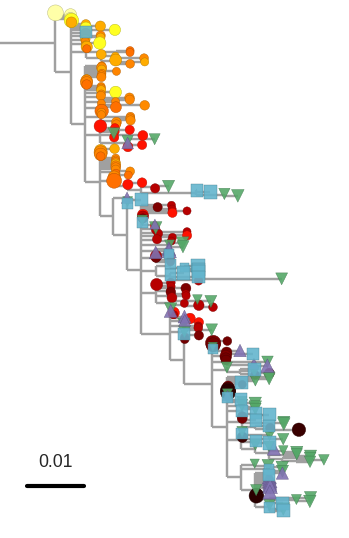
- latent HIV → barcode of a T-cell lineage
- all latent integrated virus derives from late infection
- untreated: T-cell lineages are short lived
- on therapy: T-cell clones live decades
nextstrain.org
joint work with Trevor Bedford & his lab
code at github.com/nextstrain
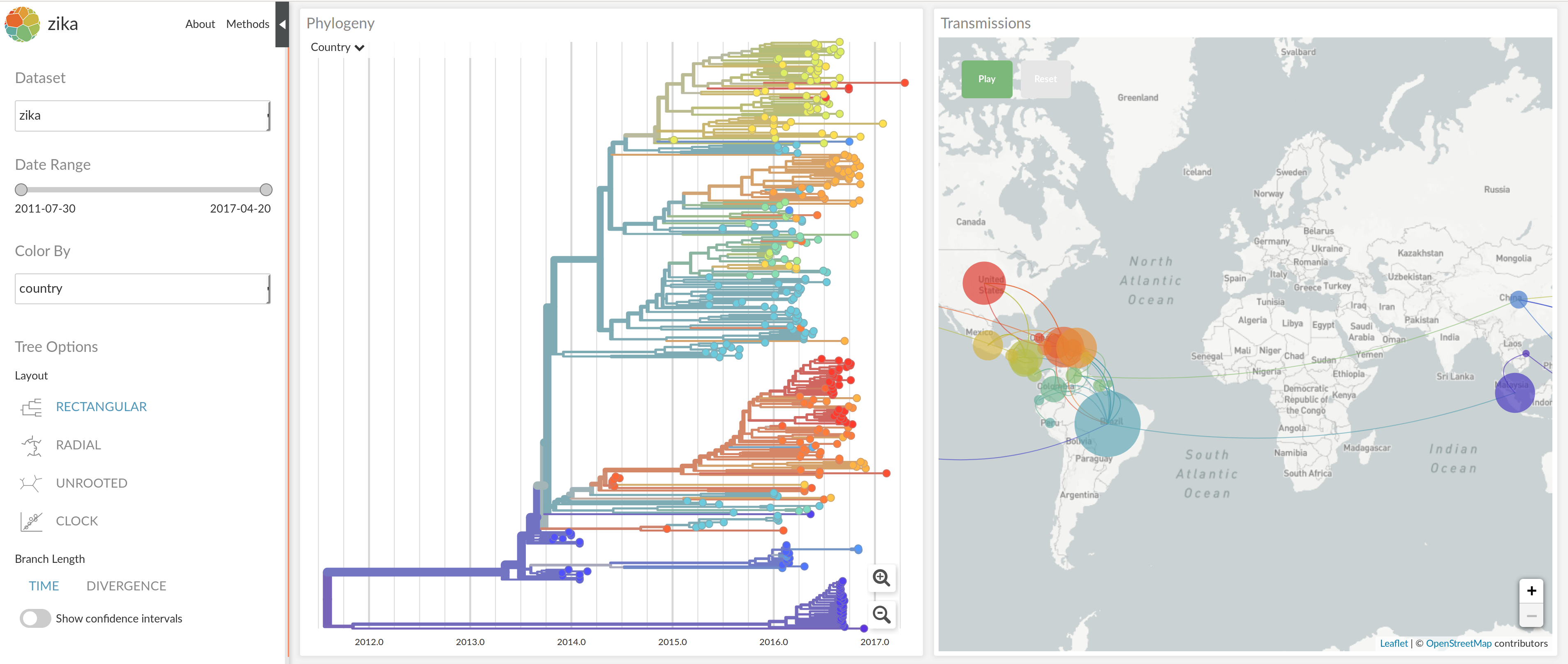
Summary
- Intra-host HIV evolution is governed by a universal fitness landscape, modulated by host-specific immune response
- Landscape of fitness costs can be estimated from intra-host diversity
- The latent HIV DNA reservoir turns over fast
- No evidence for evolution under therapy
- all data are available at hiv.biozentrum.unibas.ch
Thank you for your attention!
Acknowledgments
- Fabio Zanini
- Jan Albert
- Johanna Brodin
- Christa Lanz
- Göran Bratt
- Lina Thebo
- Vadim Puller



nextstrain.org
- Trevor Bedford
- Colin Megill
- Pavel Sagulenko
- Sidney Bell
- James Hadfield
- Wei Ding
- Tom Sibley
- Emma Hodcroft



Amplification bias and template input

Accuracy of minor variant frequencies
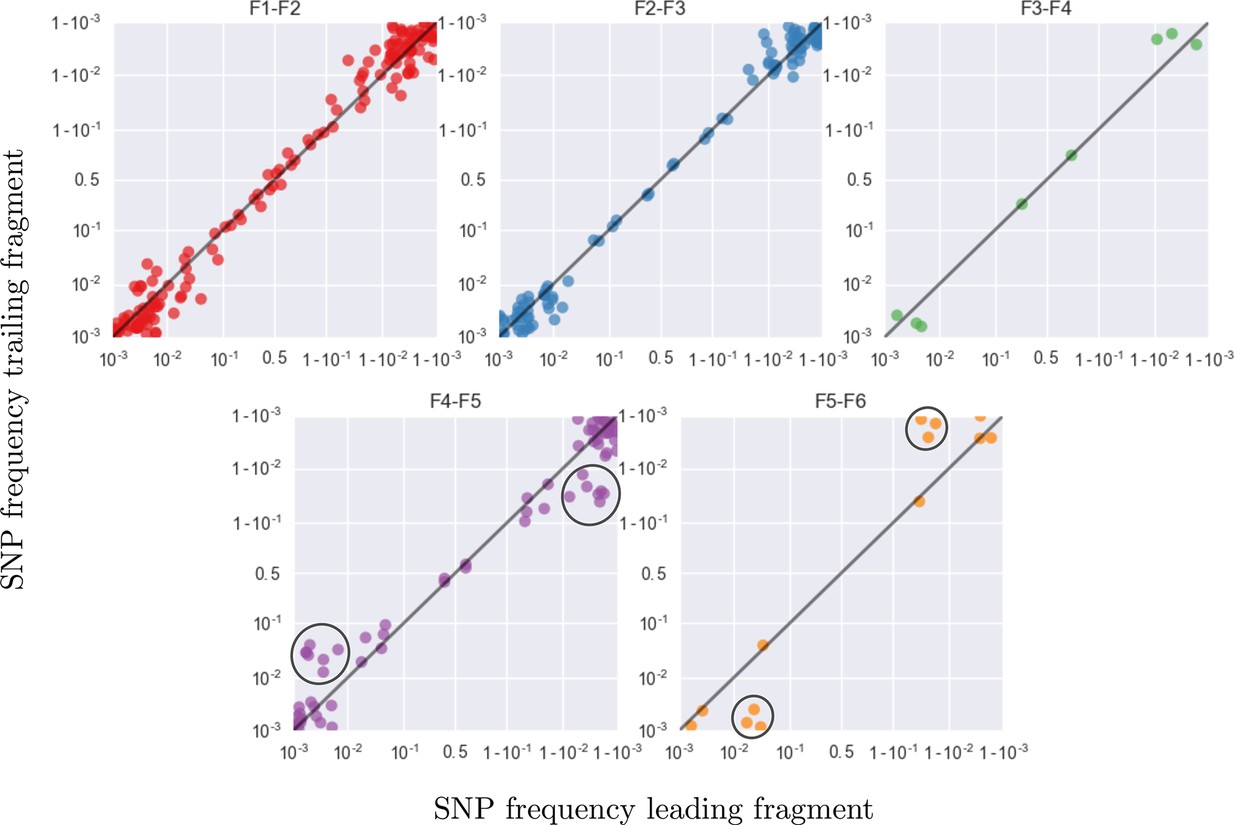
Frequency concordance in samples 4 weeks apart
The distribution of fitness costs
Zanini et al, Virus Evolution, 2017Fitness costs vs consensus amino acid
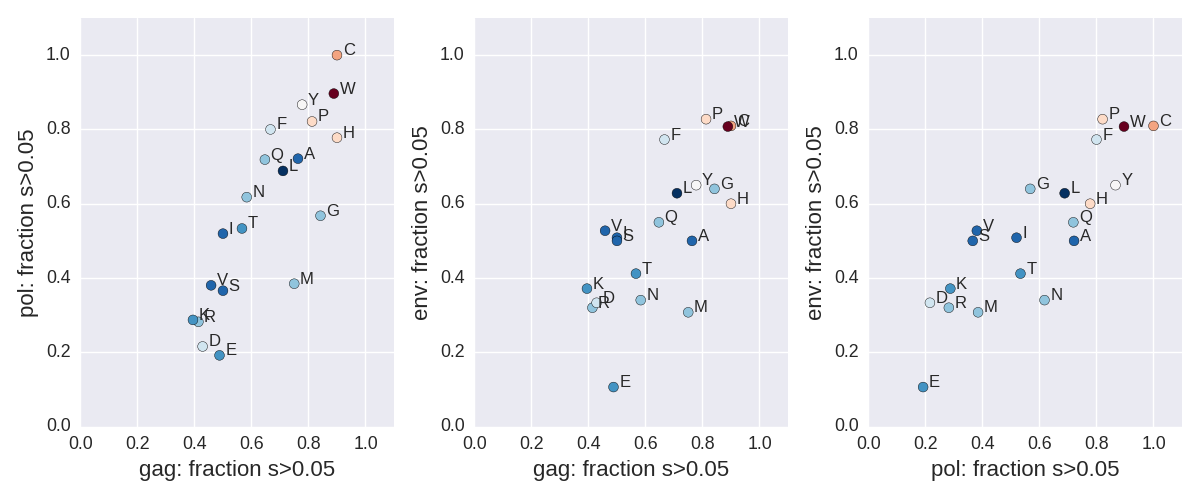 Zanini et al, Virus Evolution, 2017
Zanini et al, Virus Evolution, 2017