Real-time phylogenetic analysis of emerging pathogens
Richard Neher
Biozentrum, University of Basel
slides at neherlab.org/201810_Jena.html
Sequences record the spread of pathogens
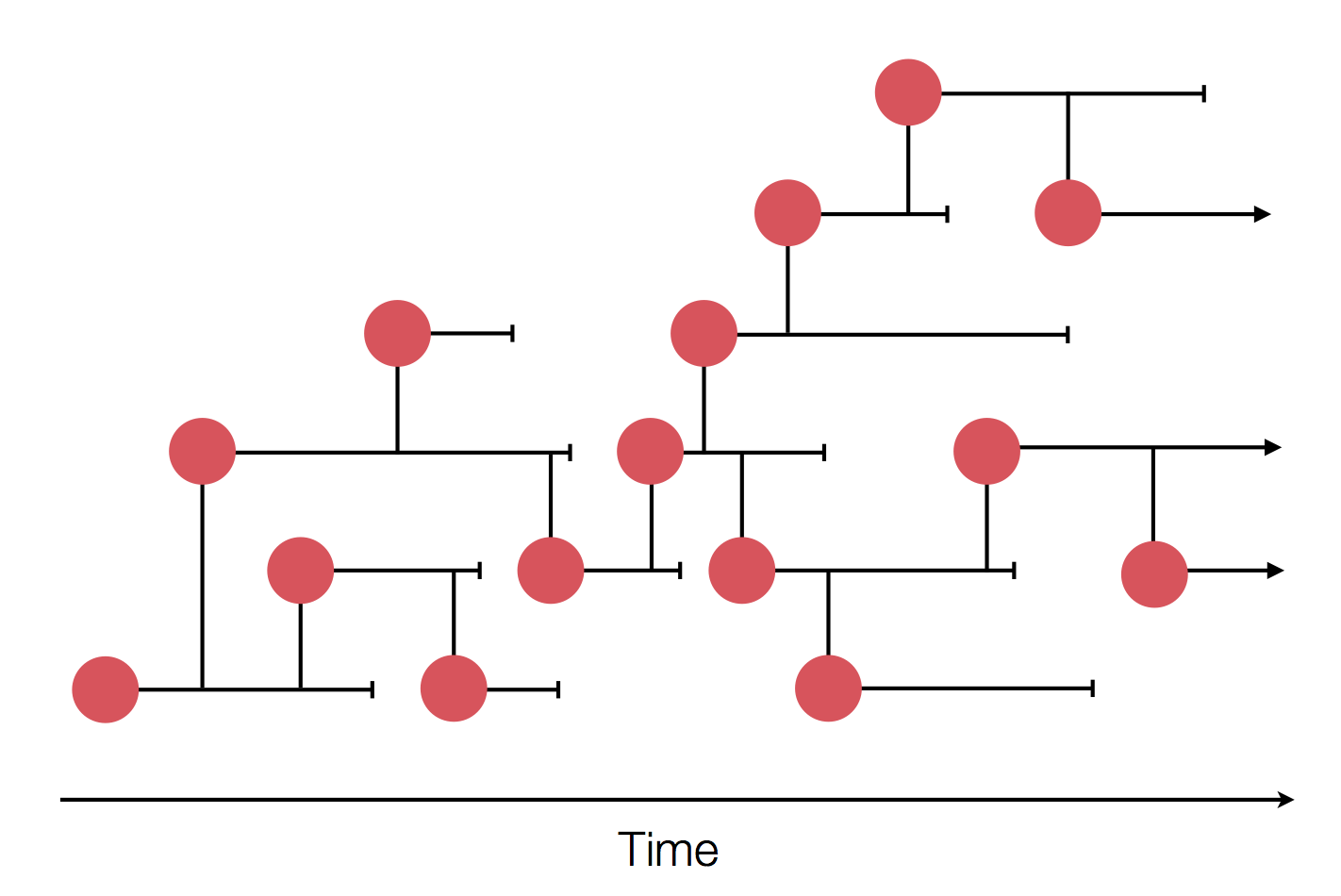
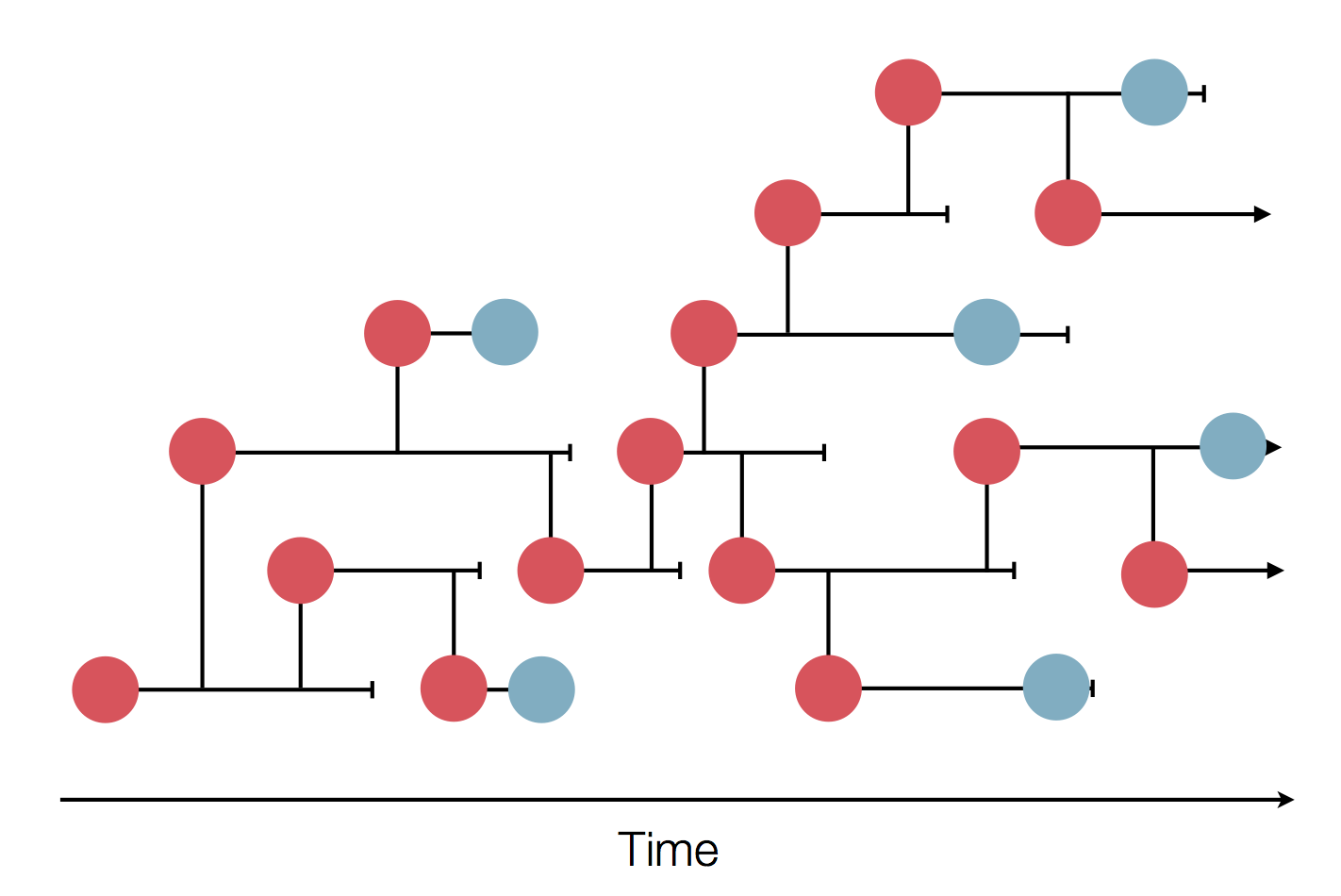
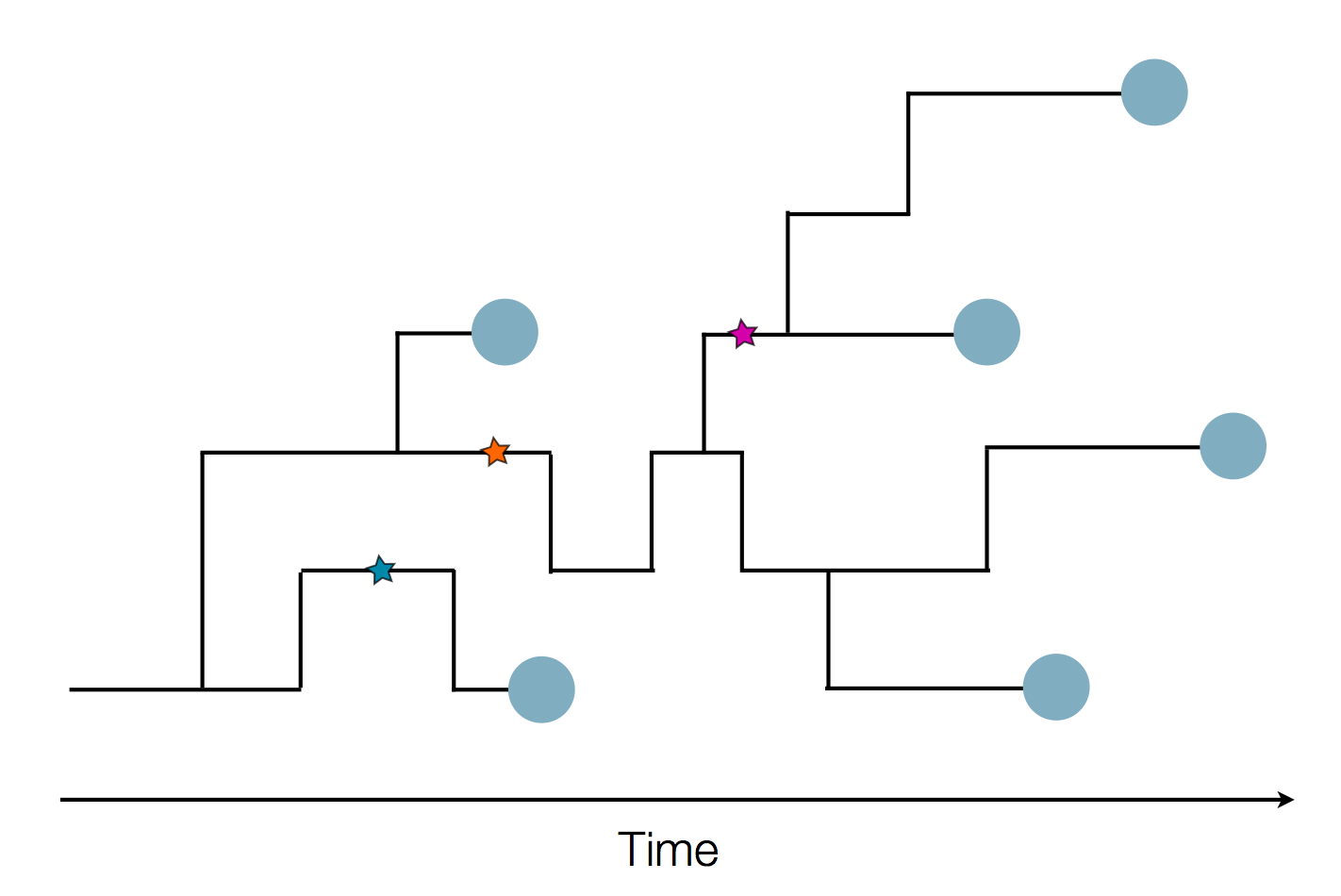
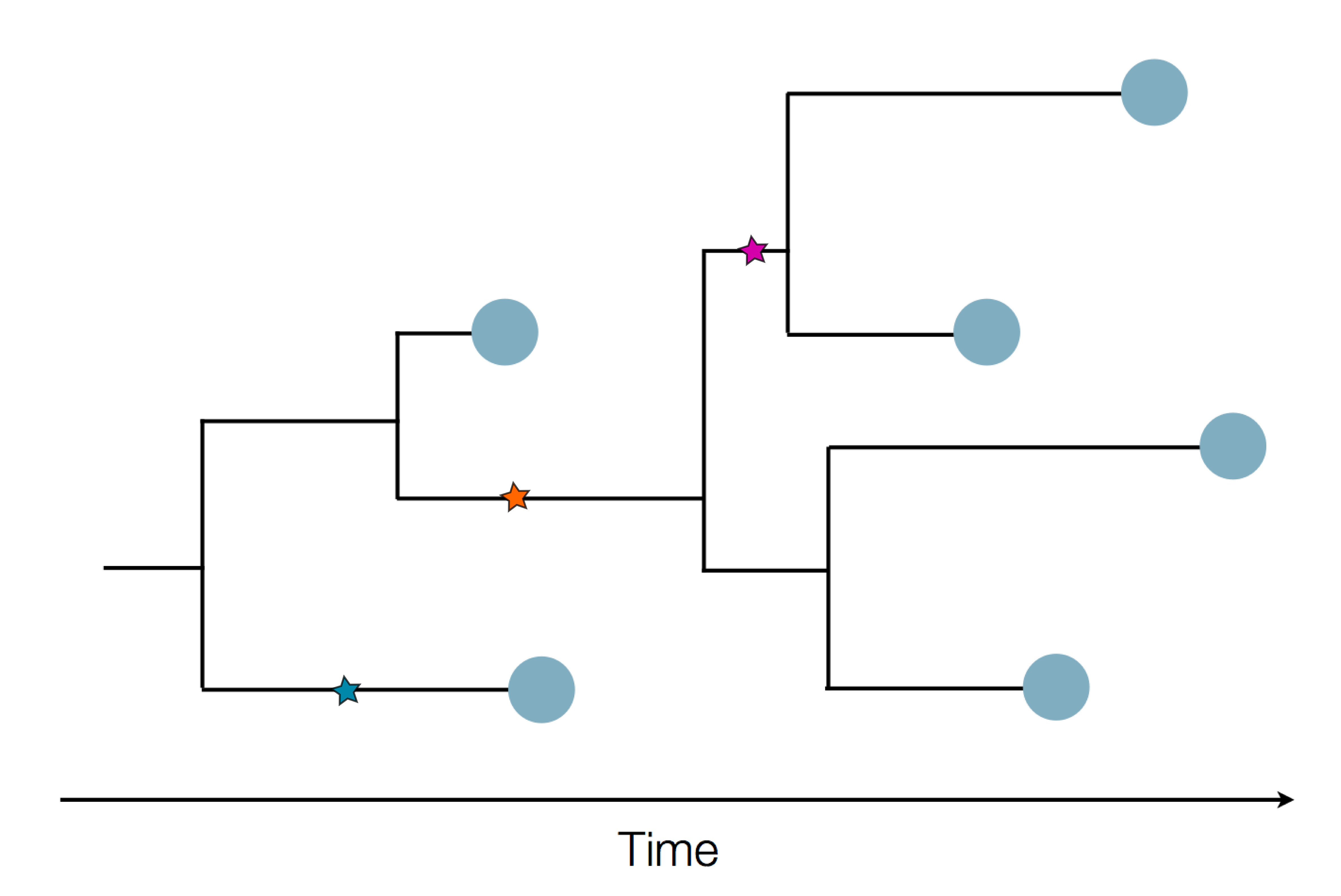
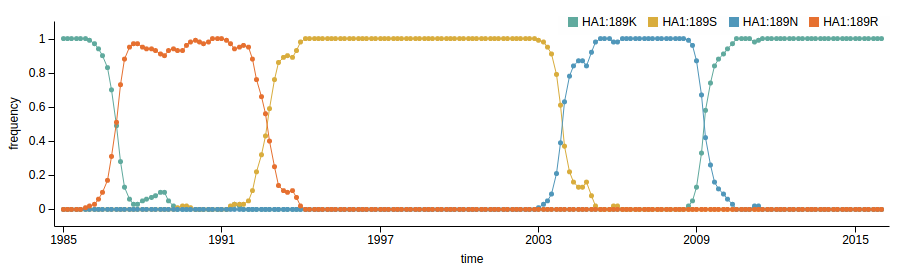
Human seasonal influenza A viruses
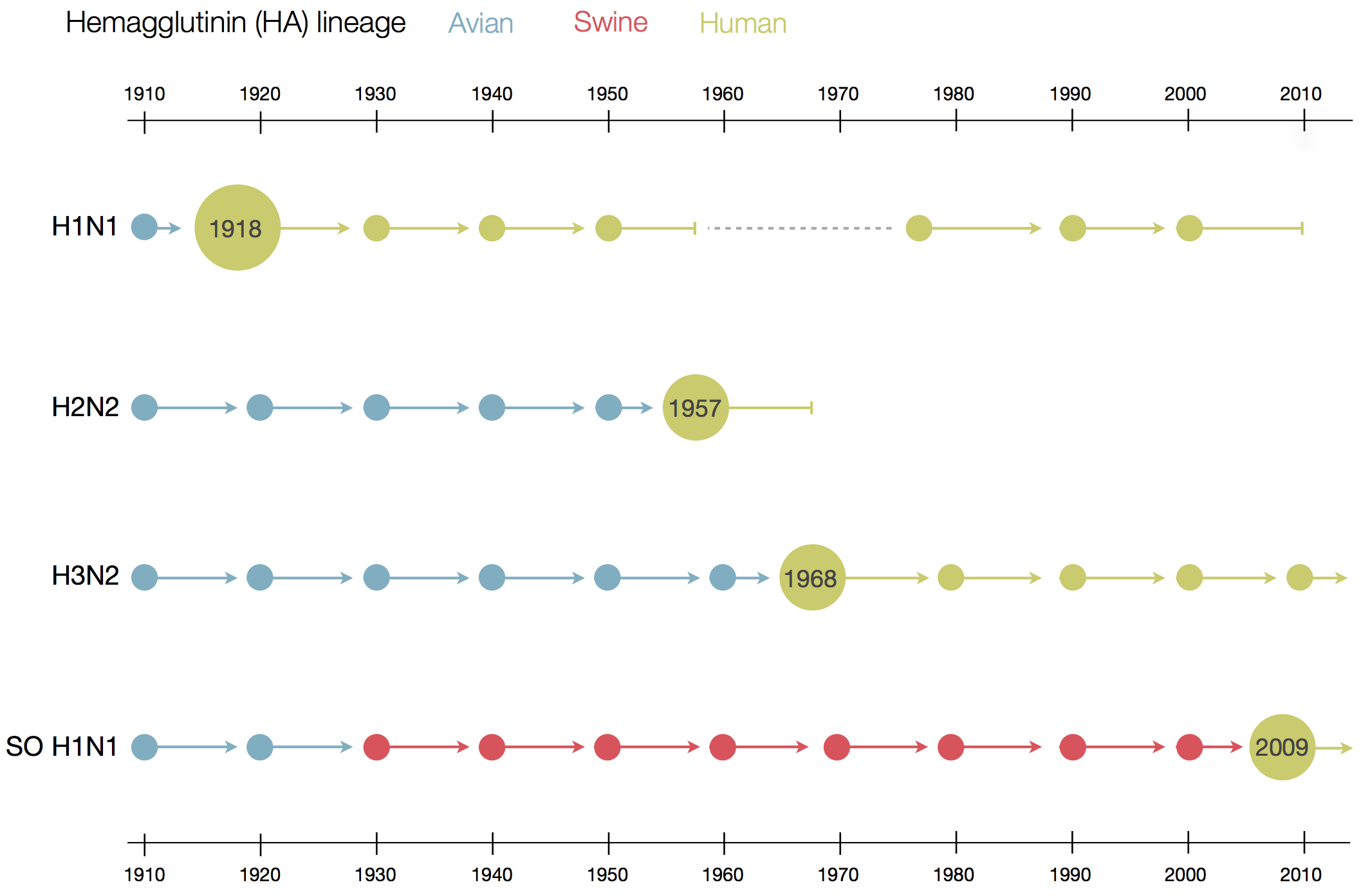
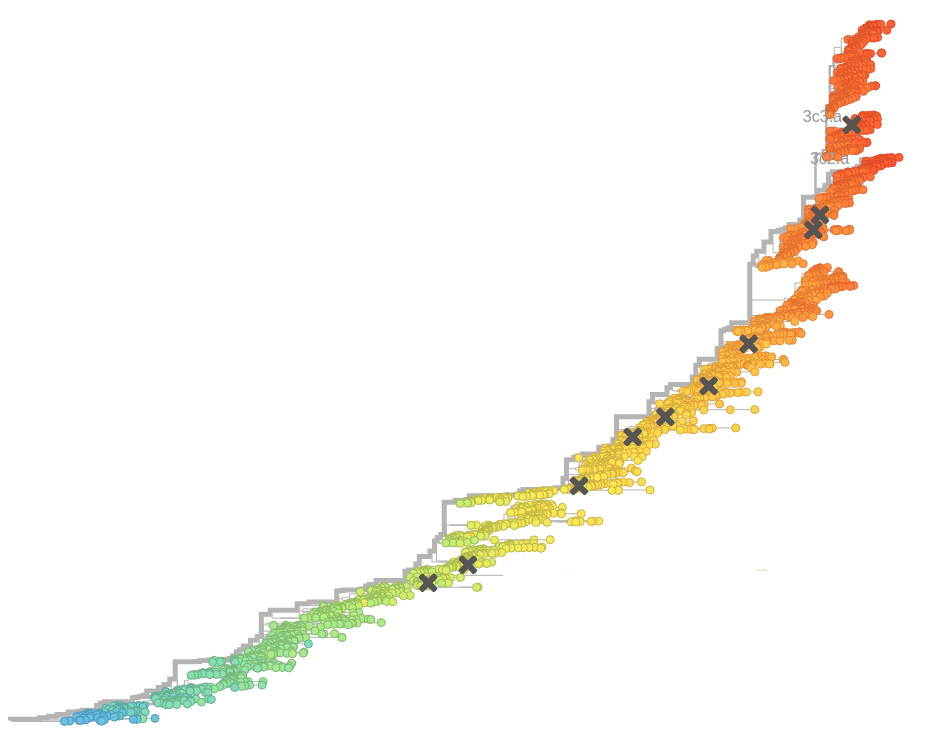
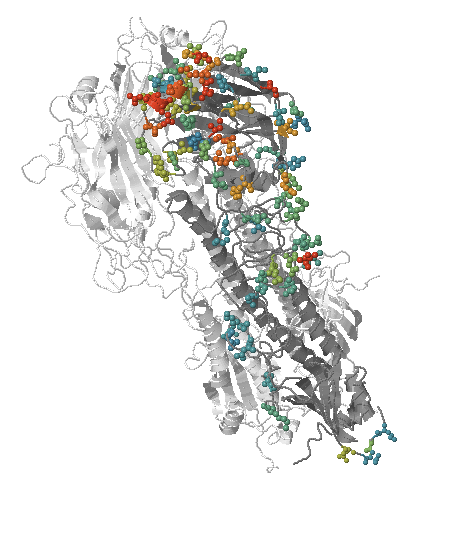
- Influenza viruses evolve to avoid human immunity
- Vaccines need frequent updates
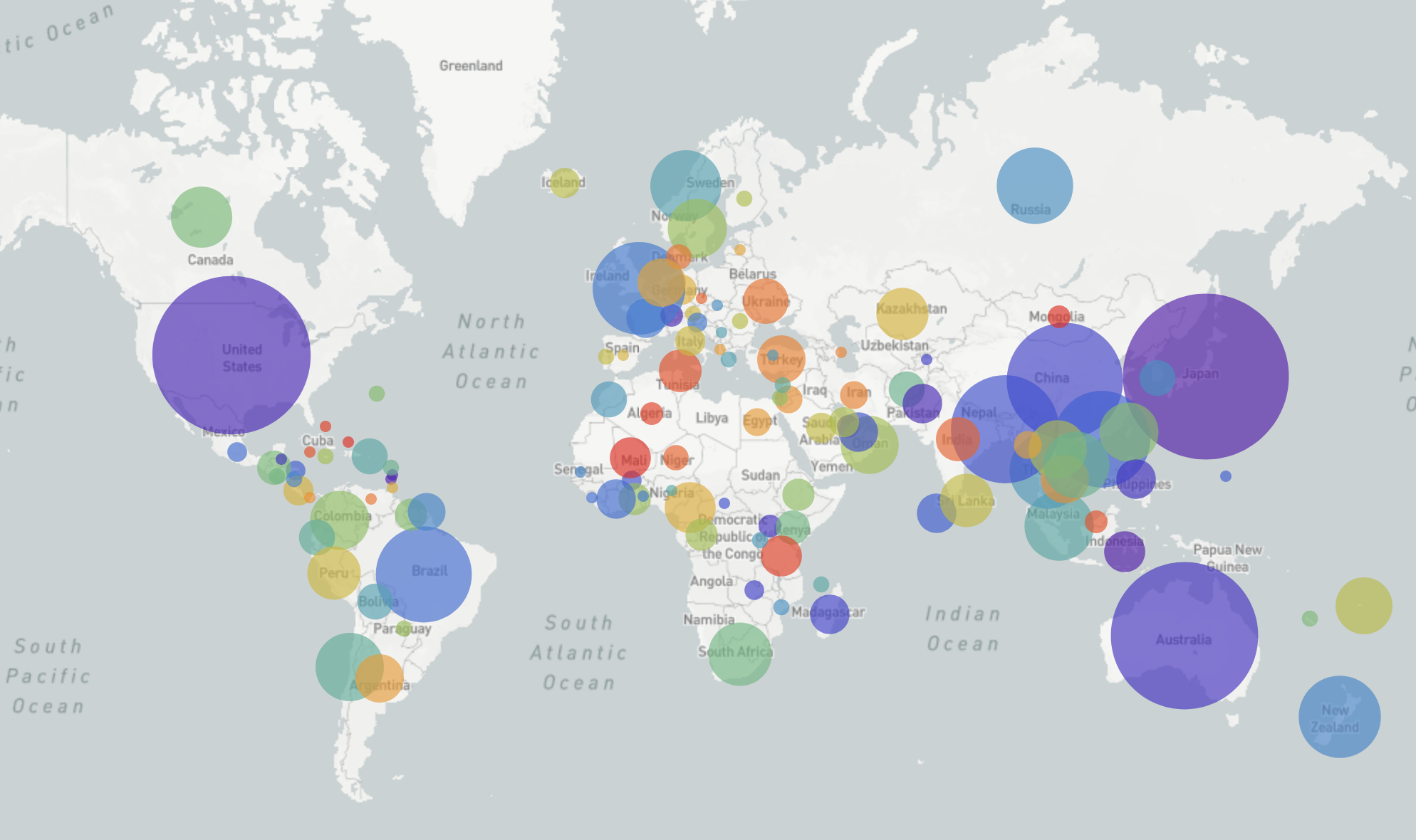
GISRS and GISAID -- Influenza virus surveillance
- comprehensive coverage of the world
- timely sharing of data -- often within 2-3 weeks of sampling
- hundreds of sequences per week (in peak months)
→ requires continuous analysis and easy dissemination
→ interpretable and intuitive visualization
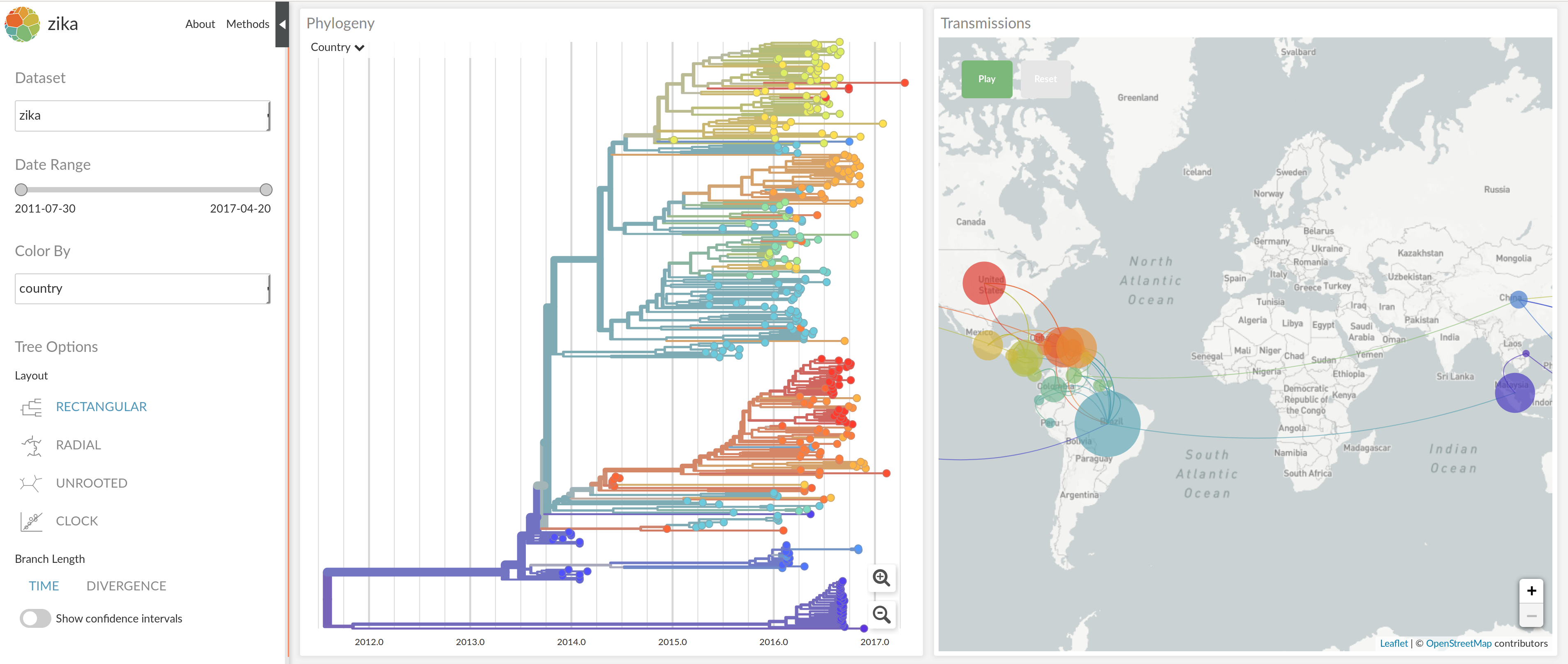
nextflu.org
joint work with Trevor Bedford & his lab
nextstrain.org
joint project with Trevor Bedford & his lab
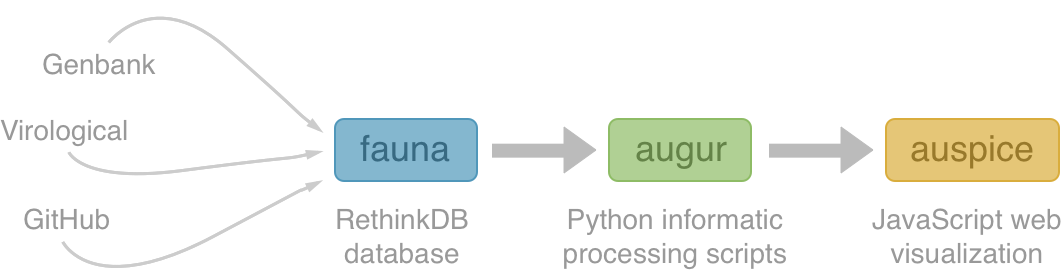
Nextstrain architecture
Using treetime to rapidly compute timetrees

Enterovirus D68 -- with Jan Albert and Robert Dyrdak
- Non-polio enterovirus
- Large outbreak in 2014 with severe neurological symptoms in young children (acute flaccid myelitis)
- Another outbreak in 2016
- Outbreaks tend to start in late summer/fall
- Several reports of EV-D68 outbreaks in the past 6 weeks
(155 AFM cases in the US as of yesterday)
Whole genome deep sequencing
- Geographic spread and phylogenetic patterns?
- Within host diversity?
- Transmission bottlenecks/multiplicity of infection?
nextstrain.org/enterovirus
joint work with Jan Albert & his lab
Phylodynamic analysis
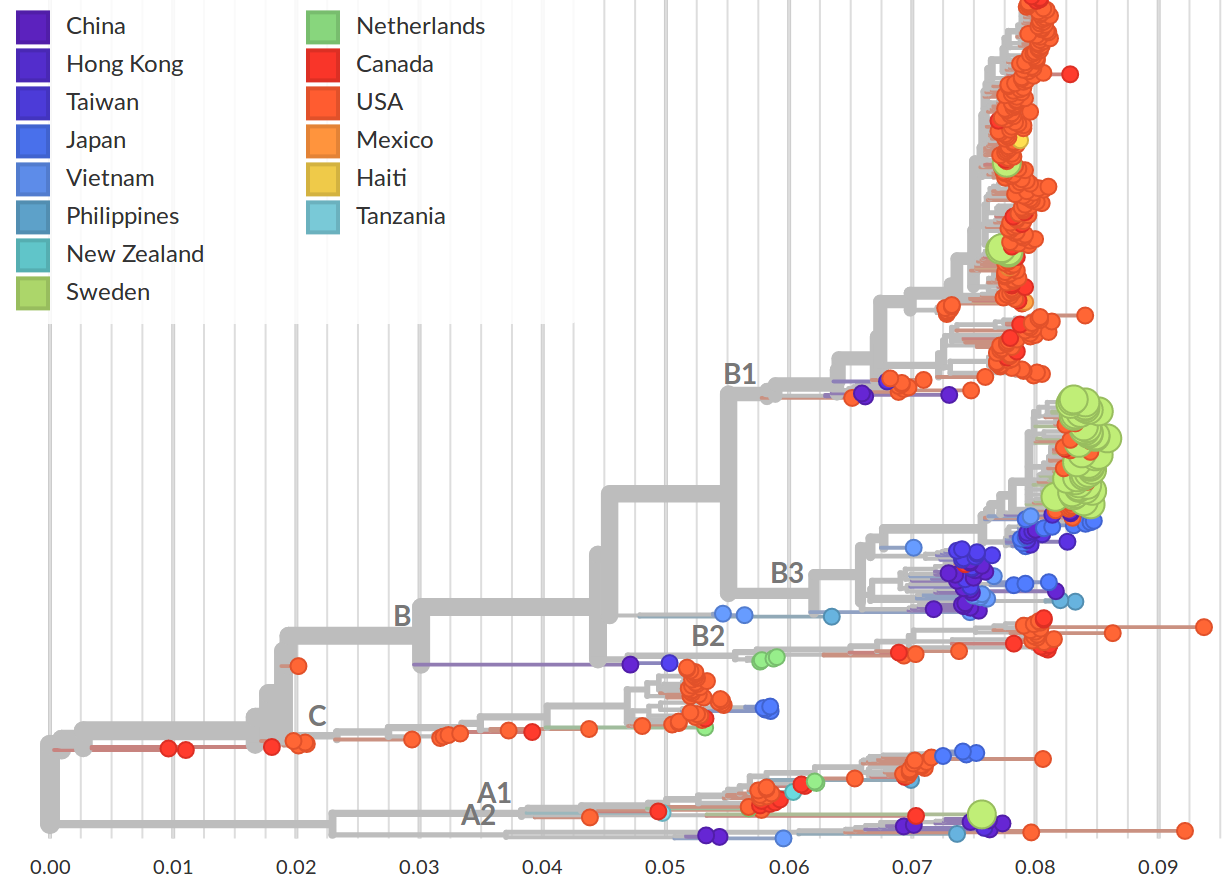
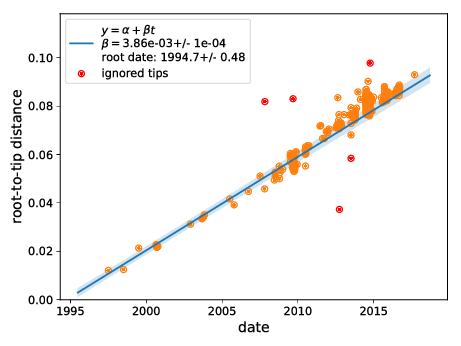
- EV-D68 outbreaks come from distinct clades.
- The evolutionary rate is very high
→ a lot of power to study transmission chains - Most variation is synonymous
Whole genome deep sequencing of Enterovirus D68
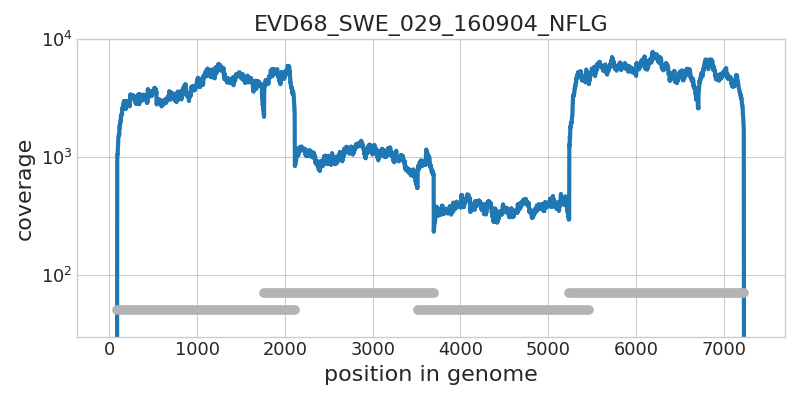
- Amplified in 4 overlapping segments
- Illumina sequenced to high coverage
iSNV frequency accuracy and sequencing errors
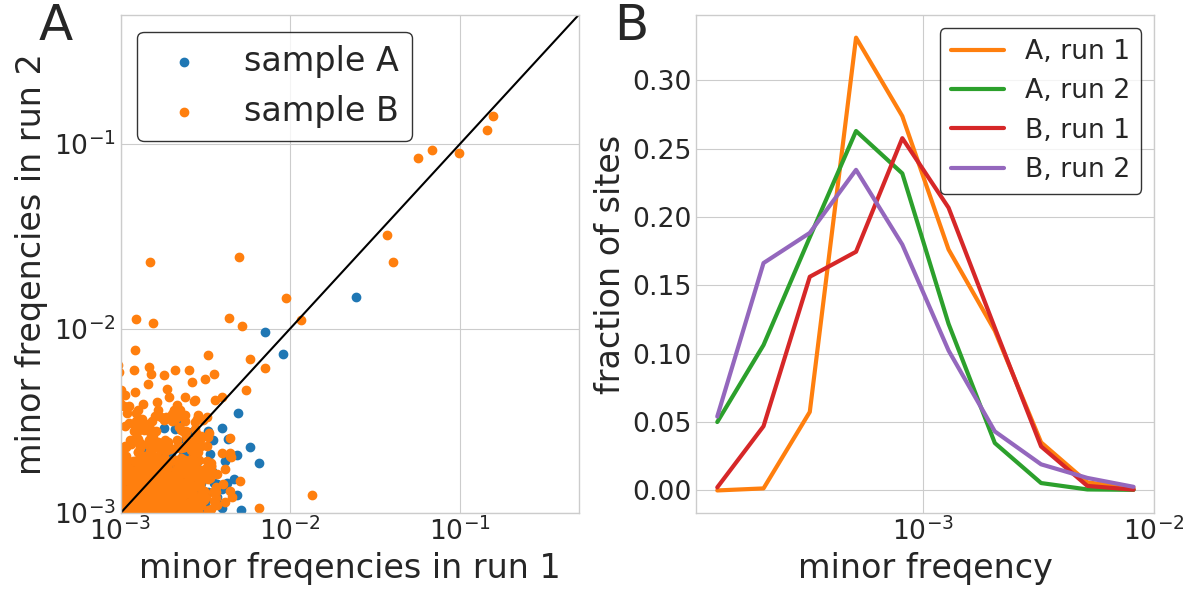
- iSNV frequencies reproducible above 1%
- background at around 1/1000
With-in host diversity
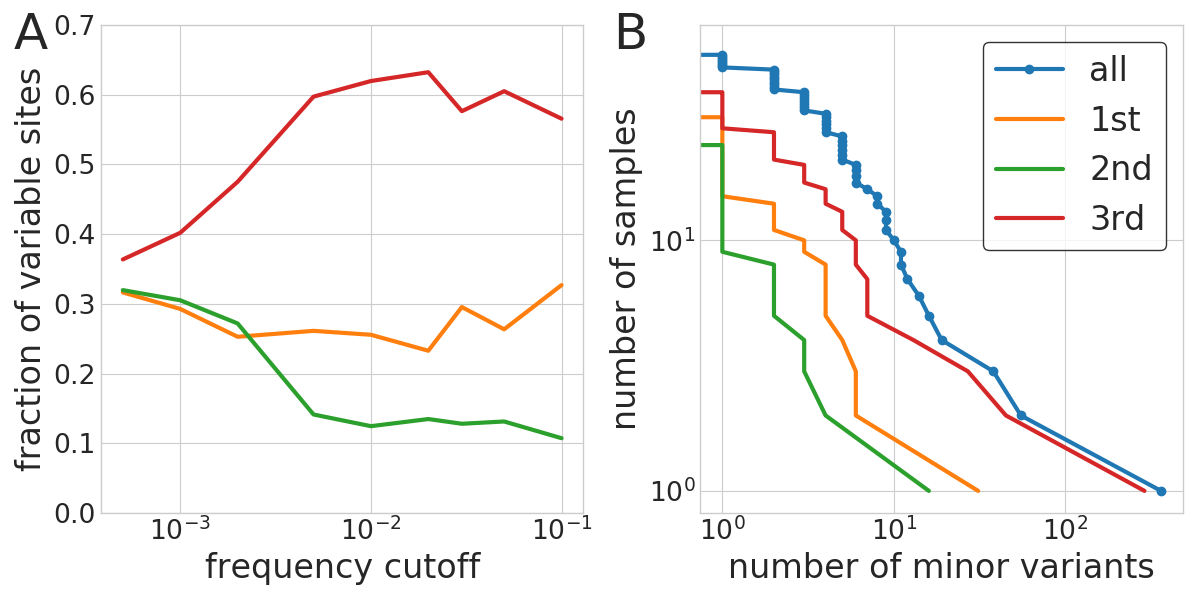
- Above 0.5%, iSNVs are biological
- Most samples have few iSNVs, three had more than 20
Dual infections
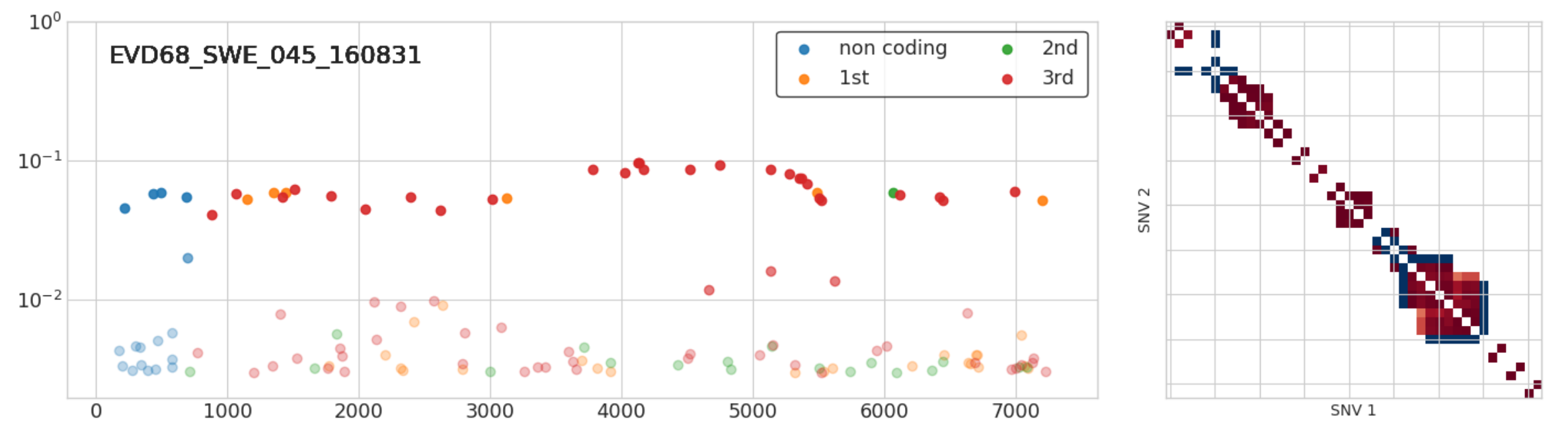
- A set of iSNVs at very similar frequencies in full linkage
- Suggest infection with two related variants
- 3 out of 50 samples: Implies high prevalence
Conclusions
- Pathogen sequence data contain information on spread and transmission
- Timely sharing is key
- Integration of sequence data with epidemiological data
- Near real-time analysis
- Dissemination of results in an intuitive yet informative way
Acknowledgments






- Trevor Bedford
- Colin Megill
- Pavel Sagulenko
- Sidney Bell
- James Hadfield
- Wei Ding
- Emma Hodcroft
- Sanda Dejanic




Acknowledgments
- Robert Dyrdak
- Jan Albert
- Lina Thebo
- Emma Hodcroft
