Universality and predictability in RNA virus evolution
Richard Neher
Biozentrum, University of Basel
slides at neherlab.org/201810_cuny.html
Human seasonal influenza viruses
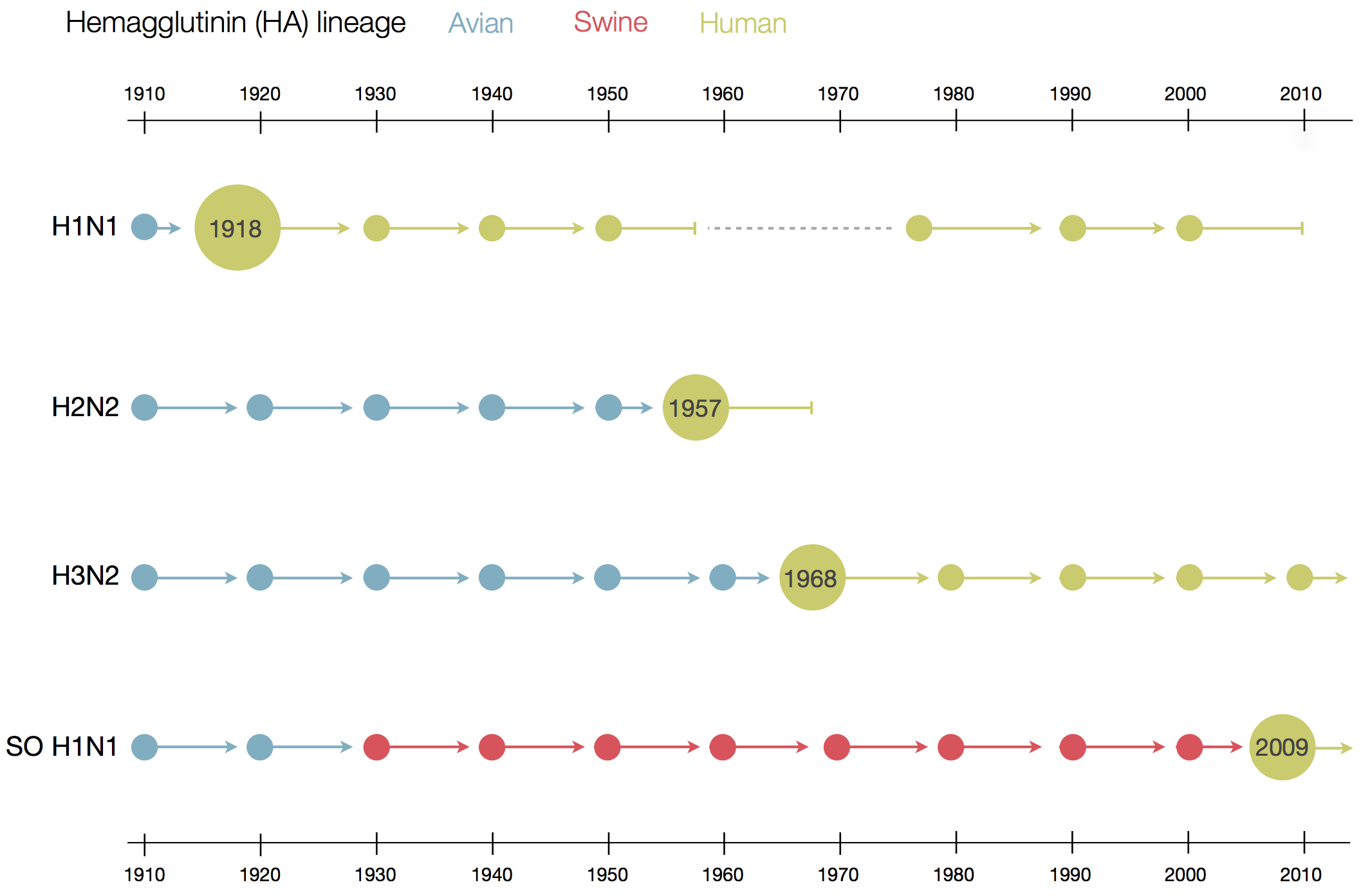
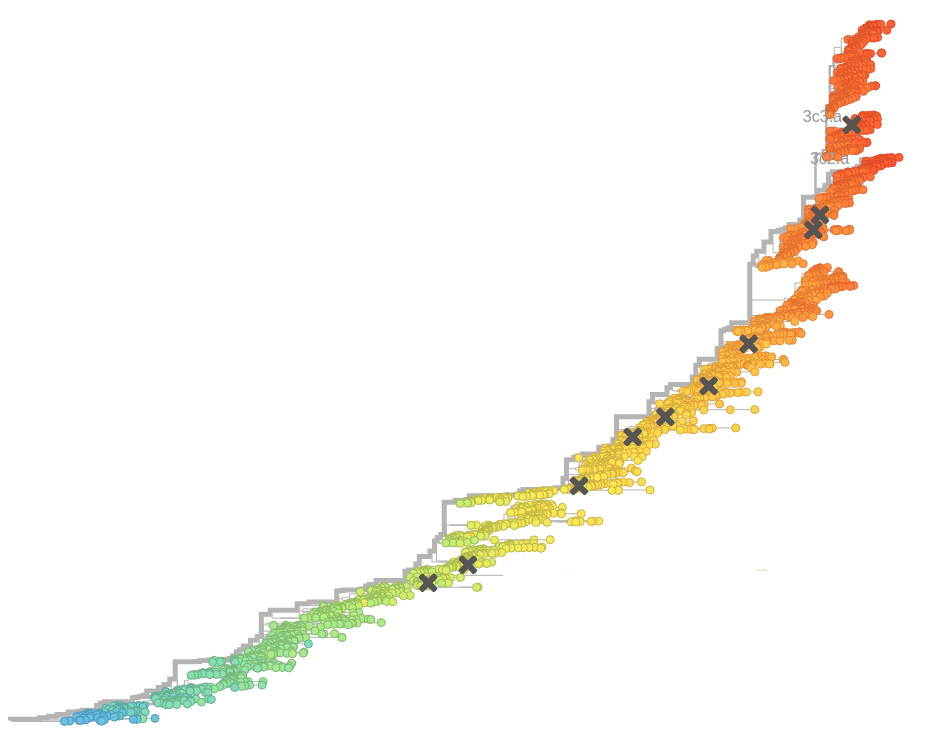
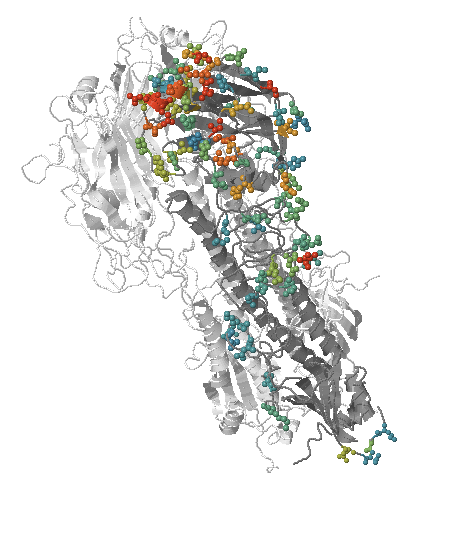
- Influenza virus evolves to avoid human immunity
- Vaccines need frequent updates
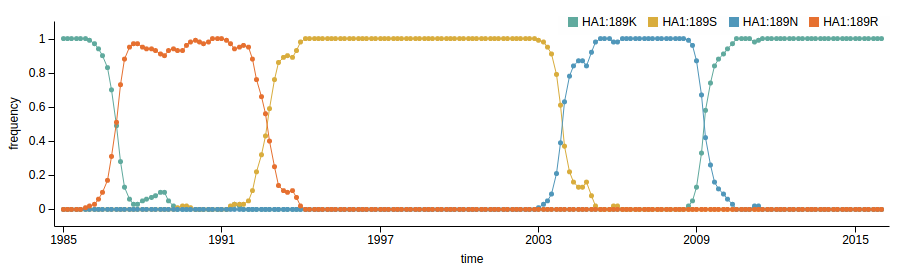
Virus evolution happens within hosts!
This is much easier to study in HIV than influenza
HIV infection
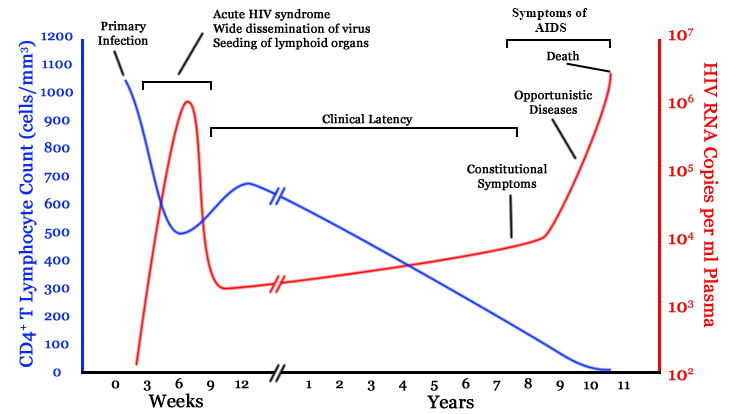
- $10^8$ cells are infected every day
- the virus repeatedly escapes immune recognition
- integrates into T-cells as
latent provirus
HIV-1 evolution within one individual
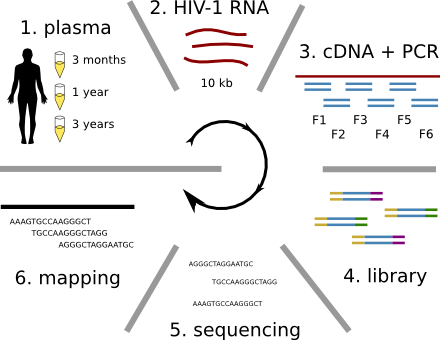

HIV-1 sequencing before and after therapy
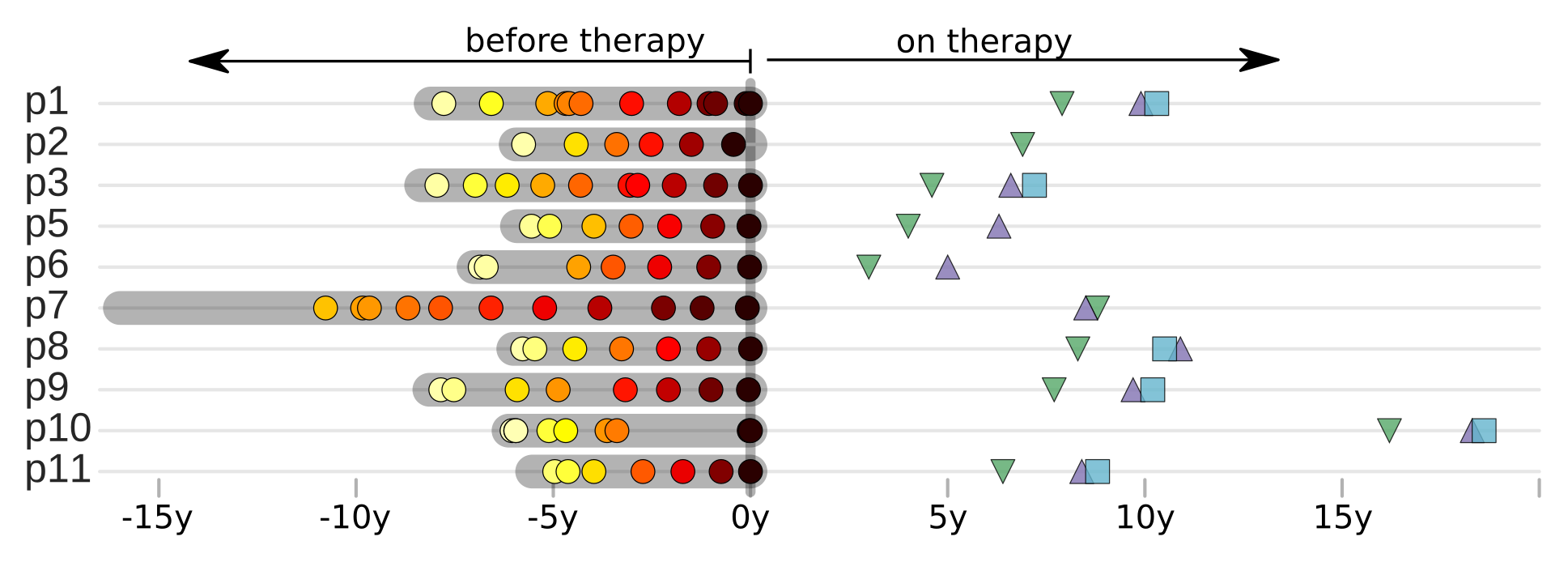 Zanini et al, eLife, 2015;
Brodin et al, eLife, 2016.
Collaboration with the group of Jan Albert
Zanini et al, eLife, 2015;
Brodin et al, eLife, 2016.
Collaboration with the group of Jan Albert
Population sequencing to track all mutations above 1%
- diverge at 0.1-1% per year
- almost whole genome coverage in 10 patients
- full data set at hiv.biozentrum.unibas.ch
Approximately neutral divergence -- silent mutations
In vivo mutation rate estimates
Divergence at increasingly conserved positions
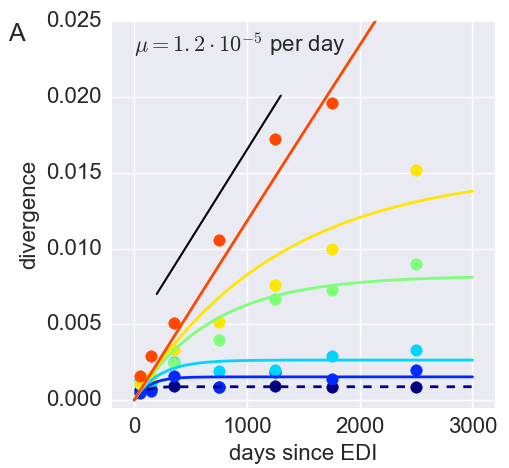
- Six categories from high to low conservation
- deleterious mutations arise with rate $\mu$
- selection against them with strength $s$
- variant frequency dynamics: $\frac{d x}{dt} = \mu -s x $
- equilibrium frequency: $\bar{x} = \mu/s $
- fitness cost: $s = \mu/\bar{x}$
- Fit model of minor variation to categories of conservation
- $\Rightarrow$ harmonic average fitness cost in category
Fitness landscape of HIV-1
Zanini et al, Virus Evolution, 2017Selection on RNA structures and regulatory sites
- Blue: all mutations
- Red: only mutations that don't change amino acids
Immune adaptation
Theory, models...?
- about 10-20 mutations fix in the HIV per year
- many of them are beneficial
- 100s of mutations at low frequencies
- most of them compromise viral replication mildly
Fitness variation in rapidly adapting populations
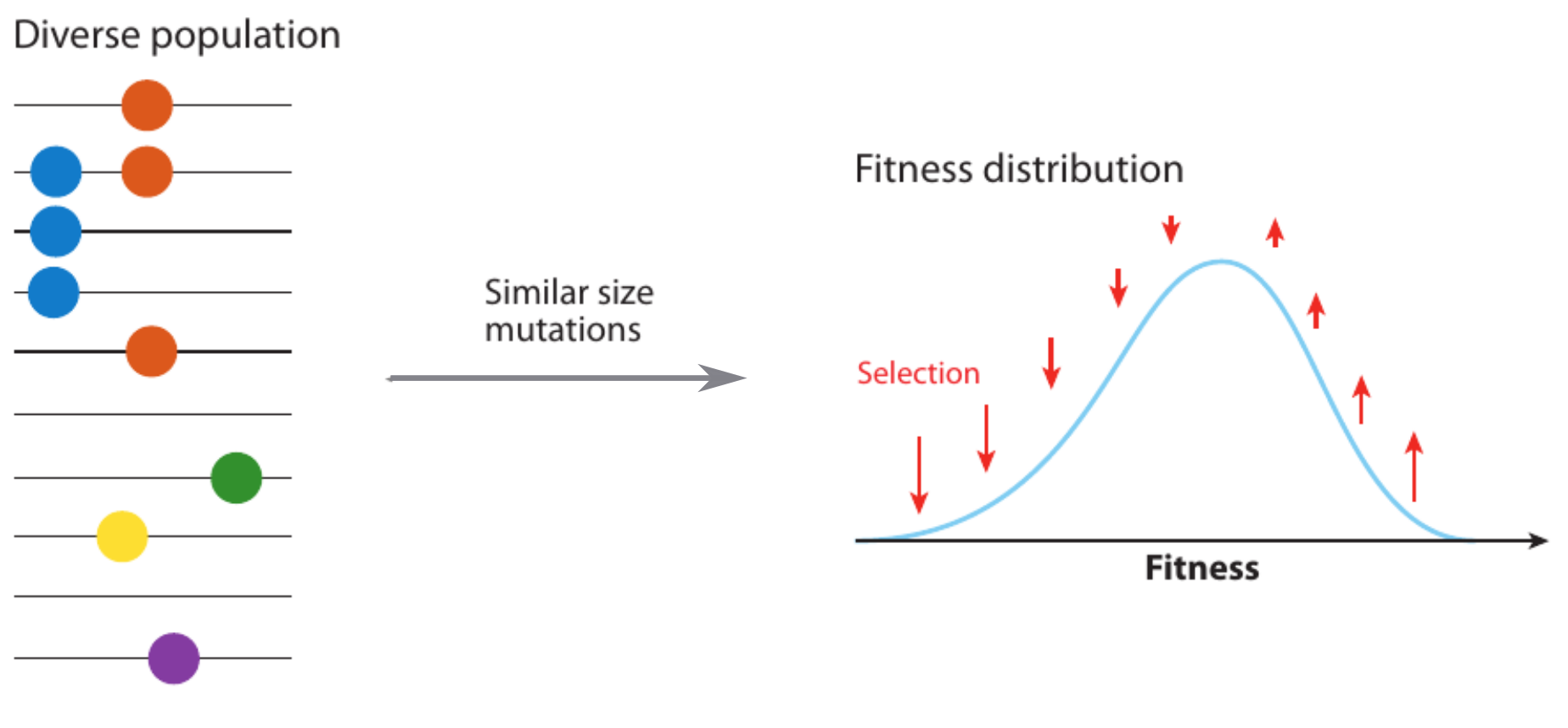
Traveling wave models of adaptation
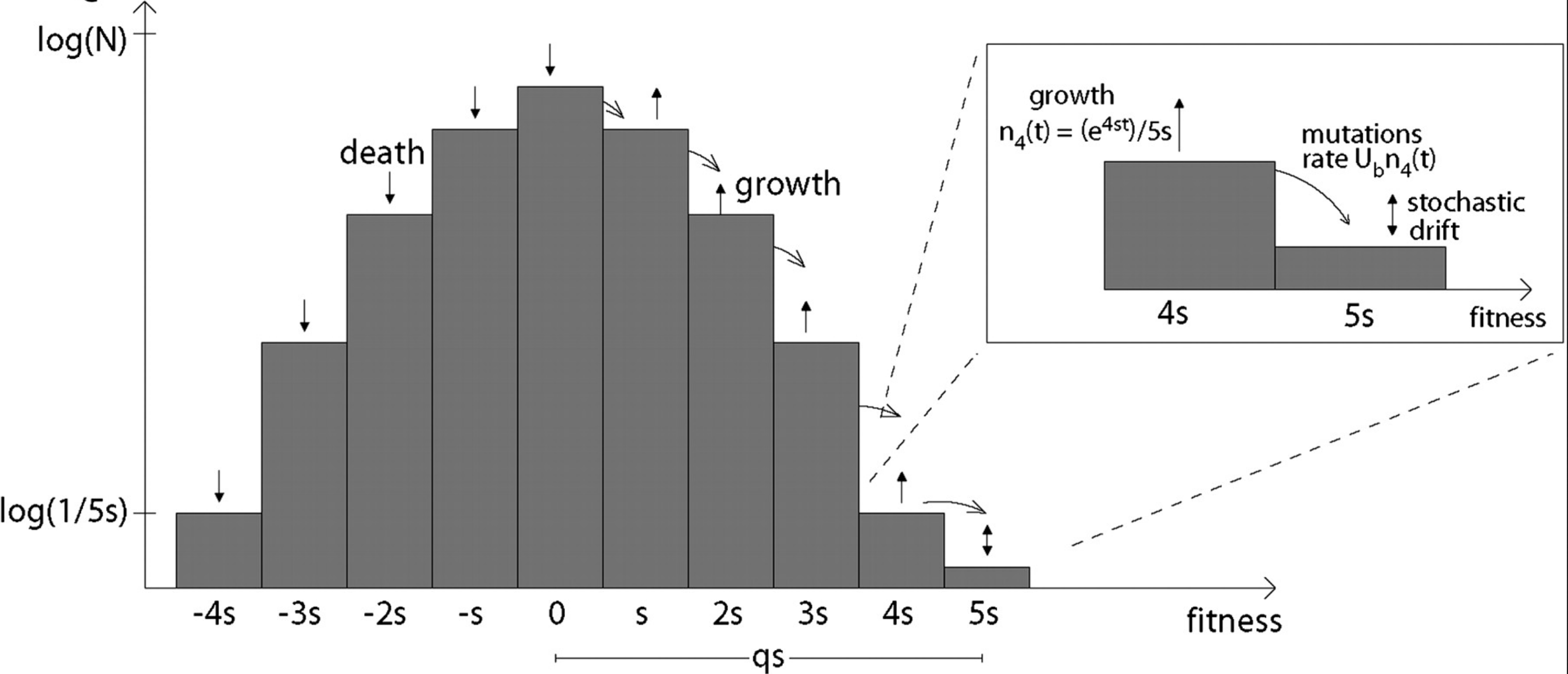
- Speed of adaptation is logarithmic in population size
- Environment (fitness landscape), not mutation supply, determines adaptation
- Different models have universal emerging properties
Dynamics, genetic diversity, and phylogenetic trees
evolutionary processes ↔ trees ↔ genetic diversity
Neutral/Kingman coalescent
strong selection
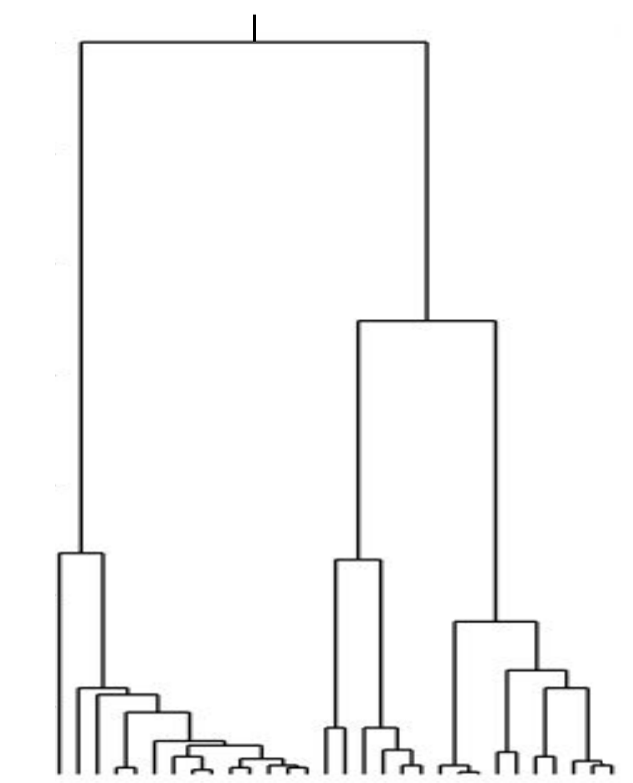
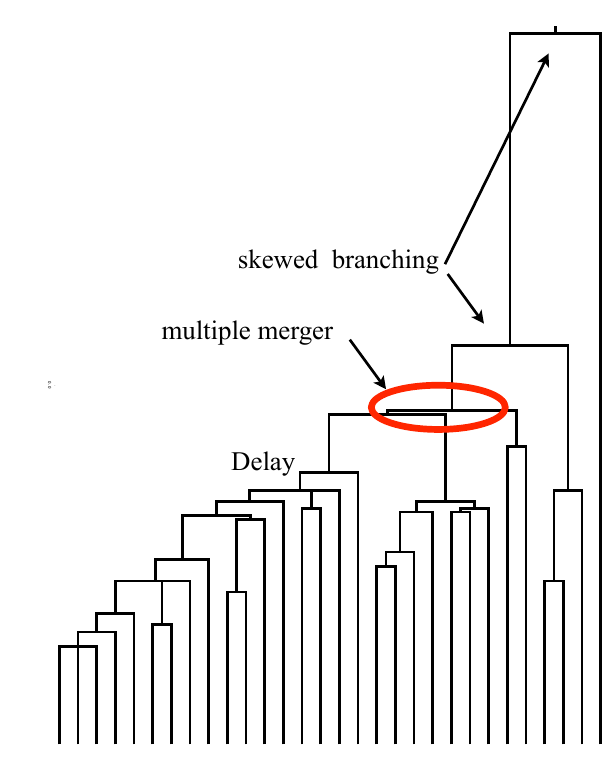
Bolthausen-Sznitman Coalescent
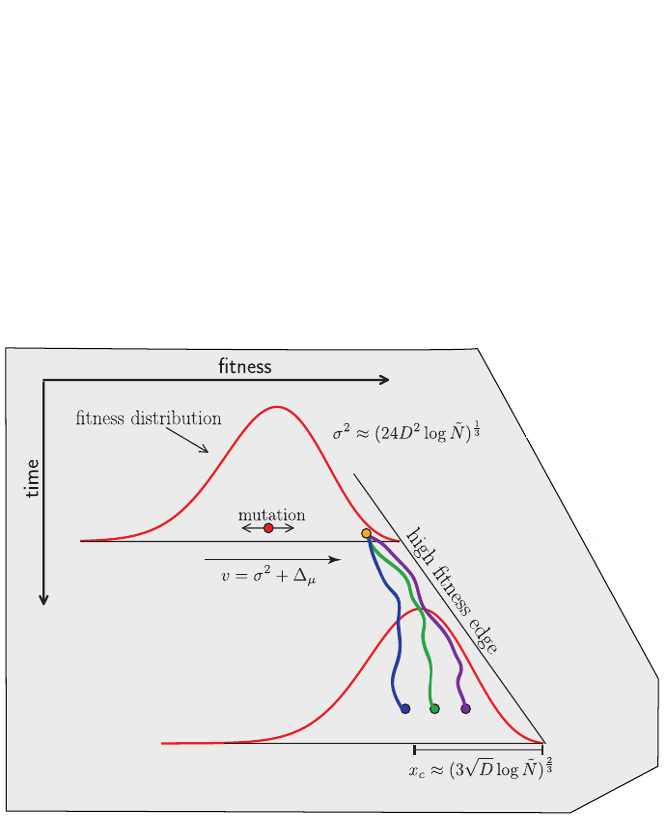
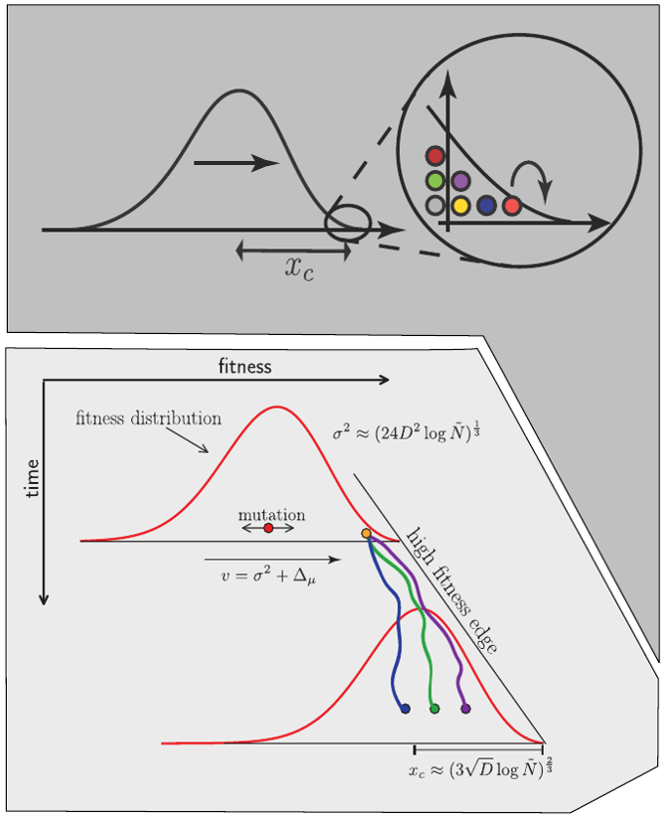
Traveling waves and the Bolthausen-Snitman coalescent
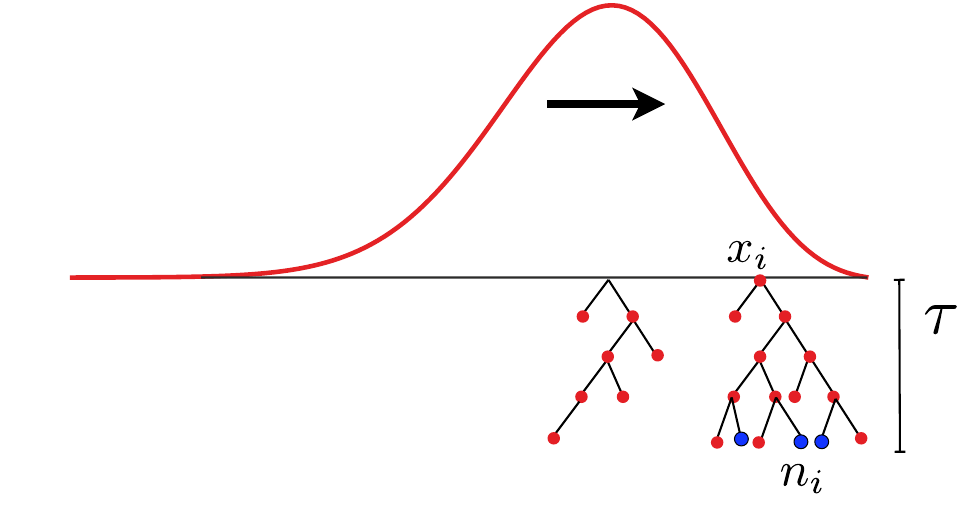
- Branching process approximation: $P(n_i, t|x_i)$
-
Does a sample (blue dots) have a common ancestor $\tau$ generations ago?
$\quad Q_b = \langle \sum_i \left(\frac{n_i}{\sum_j n_j}\right)^b\rangle \approx \frac{\tau-T_c}{T_c(b-1)} $
U-shaped polarized site frequency spectra
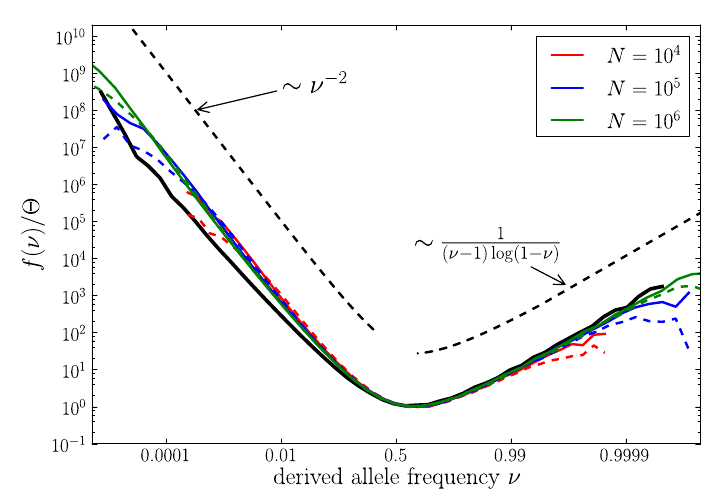

Bursts in a tree ↔ high fitness genotypes
Can we read fitness of a tree?
nextflu.org
joint work with Trevor Bedford & his lab
Predicting evolution
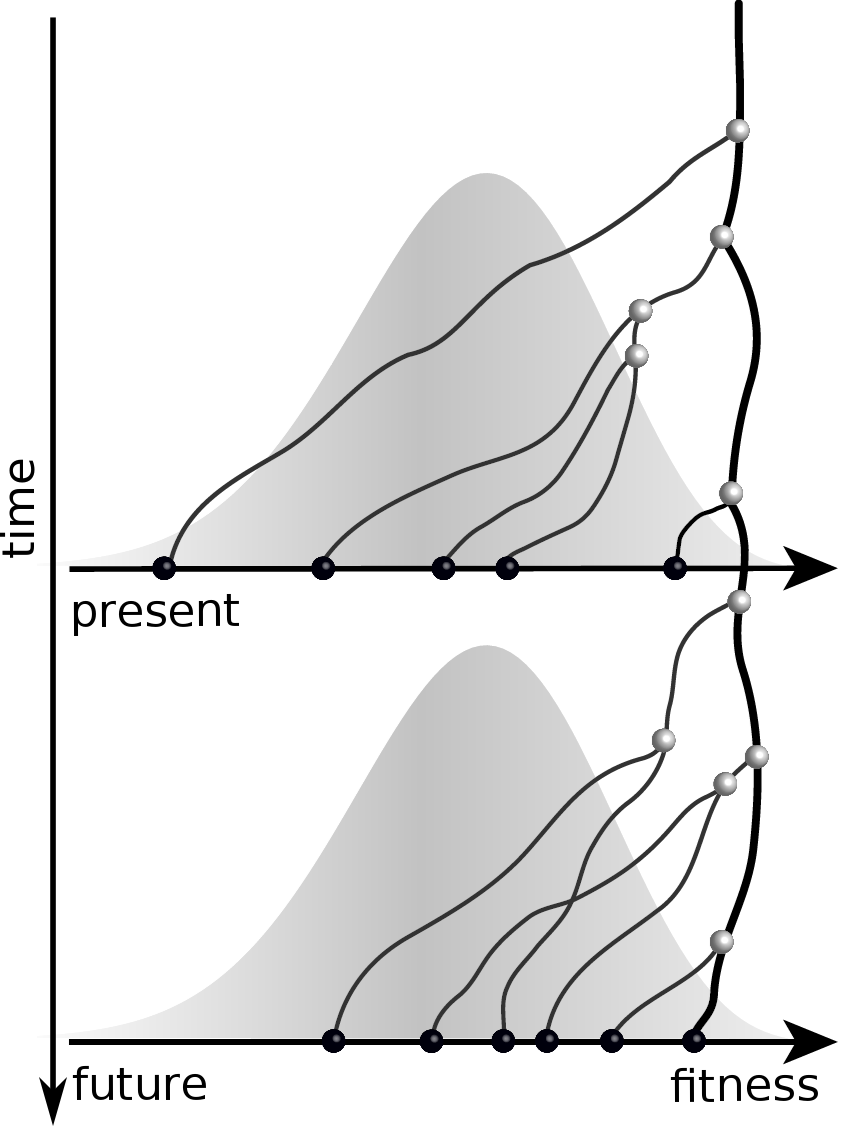
Given the branching pattern:
- can we predict fitness?
- pick the closest relative of the future?
Fitness inference from trees
$$P(\mathbf{x}|T) = \frac{1}{Z(T)} p_0(x_0) \prod_{i=0}^{n_{int}} g(x_{i_1}, t_{i_1}| x_i, t_i)g(x_{i_2}, t_{i_2}| x_i, t_i)$$
RN, Russell, Shraiman, eLife, 2014
Validate on simulation data
- simulate evolution
- sample sequences
- reconstruct trees
- infer fitness
- predict ancestor of future
- compare to truth

Validation on simulated data
Prediction of the dominating H3N2 influenza strain
- no influenza specific input
- how can the model be improved? (see model by Luksza & Laessig)
- what other context might this apply?
Summary
- RNA virus evolution can be observed directly
- Extensive reversion to preferred amino acid sequence
- Rapidly adapting population require new population genetic models
- Those model can be used to infer fit clades
- Future influenza population can be anticipated
- Automated real-time analysis can help fight the spread of disease
Acknowledgments
- Fabio Zanini
- Jan Albert
- Johanna Brodin
- Christa Lanz
- Göran Bratt
- Lina Thebo
- Vadim Puller



Acknowlegdements






- Trevor Bedford
- Colin Megill
- Pavel Sagulenko
- Sidney Bell
- James Hadfield
- Wei Ding
- Emma Hodcroft
- Sanda Dejanic




Amplification bias and template input

Accuracy of minor variant frequencies
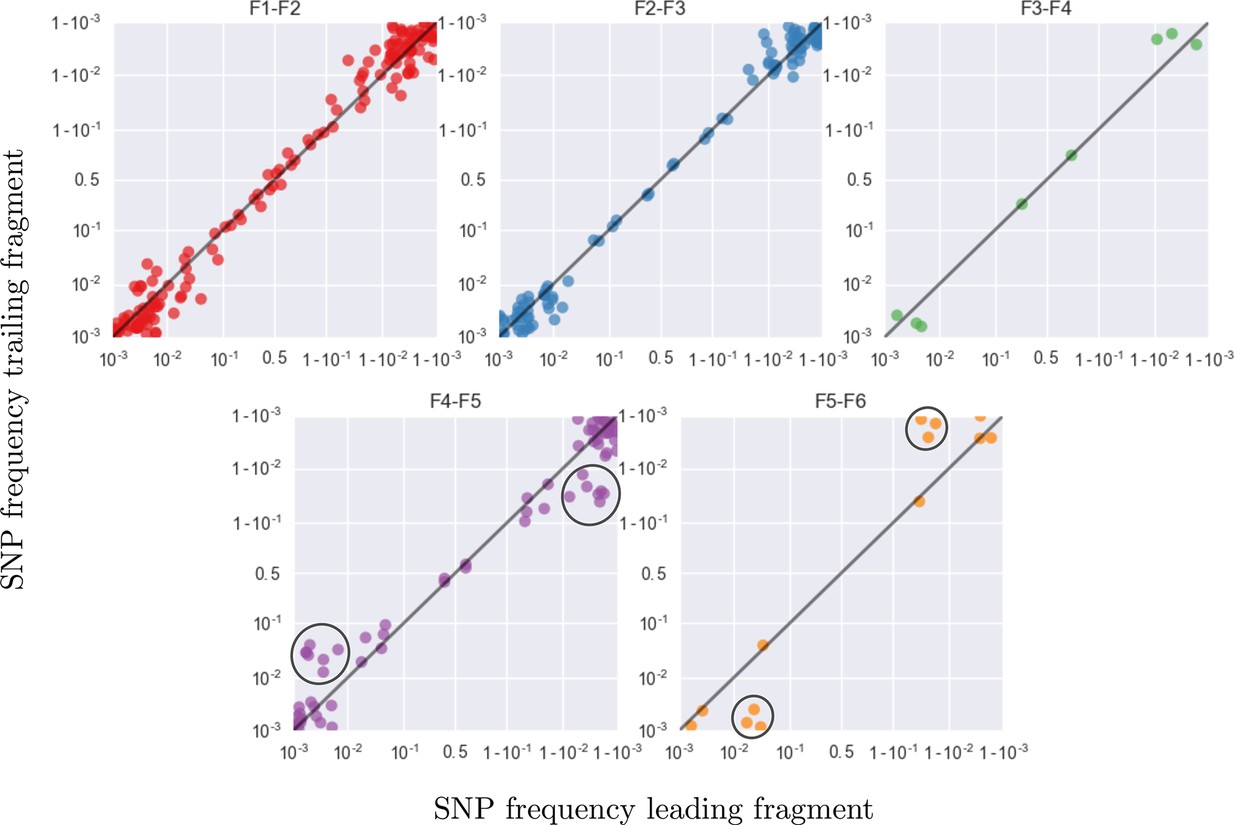
Frequency concordance in samples 4 weeks apart
The distribution of fitness costs
Zanini et al, Virus Evolution, 2017Fitness costs vs consensus amino acid
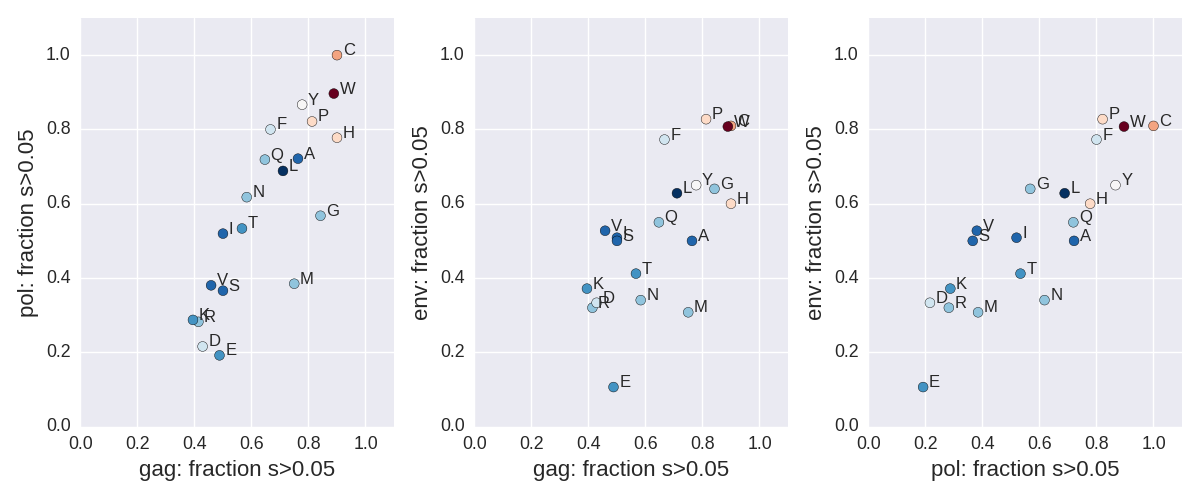 Zanini et al, Virus Evolution, 2017
Zanini et al, Virus Evolution, 2017
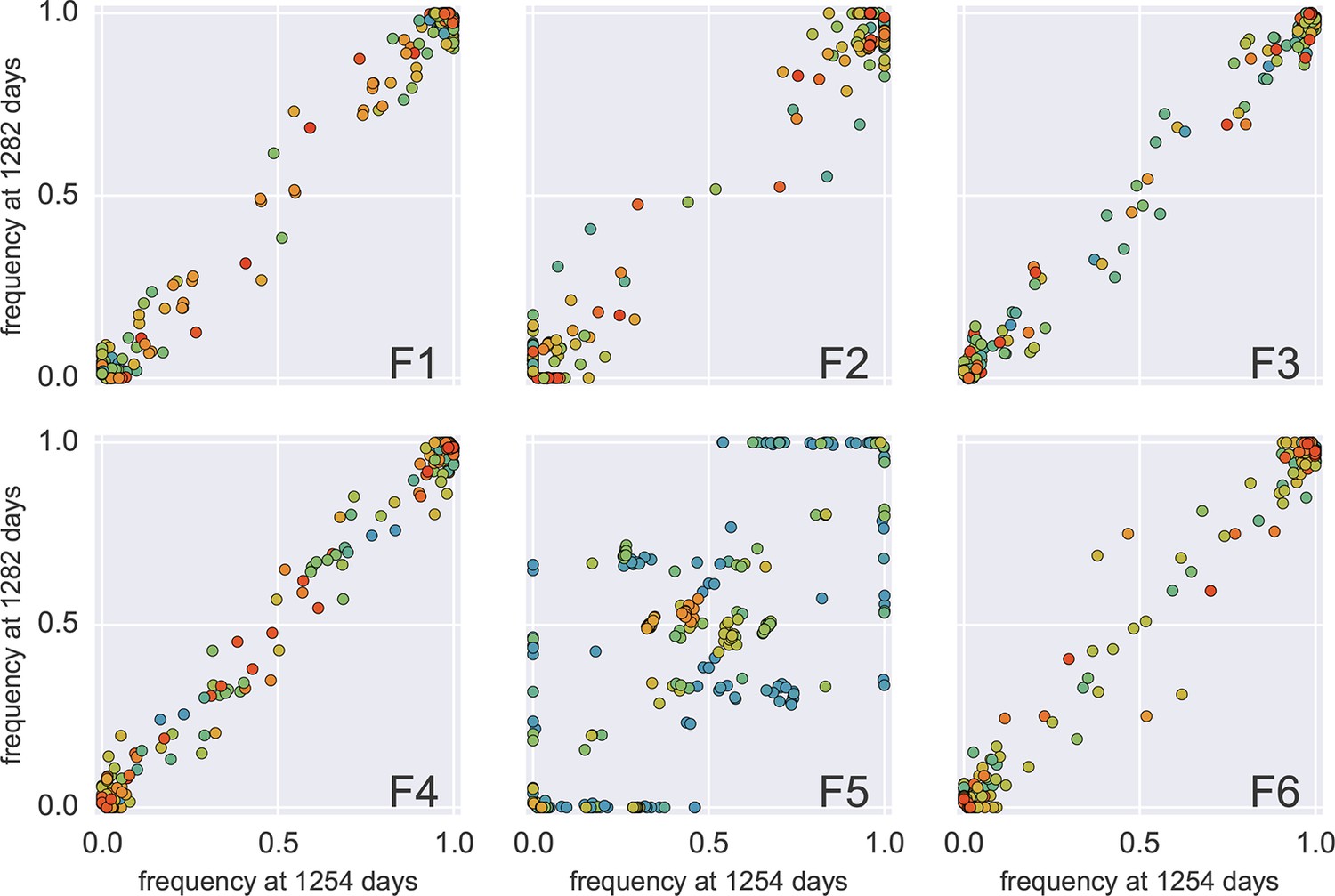
Frequent reversion of previously beneficial mutations
- HIV escapes immune systems
- most mutations are costly
- humans selects for different mutations
- compensation or reversion?
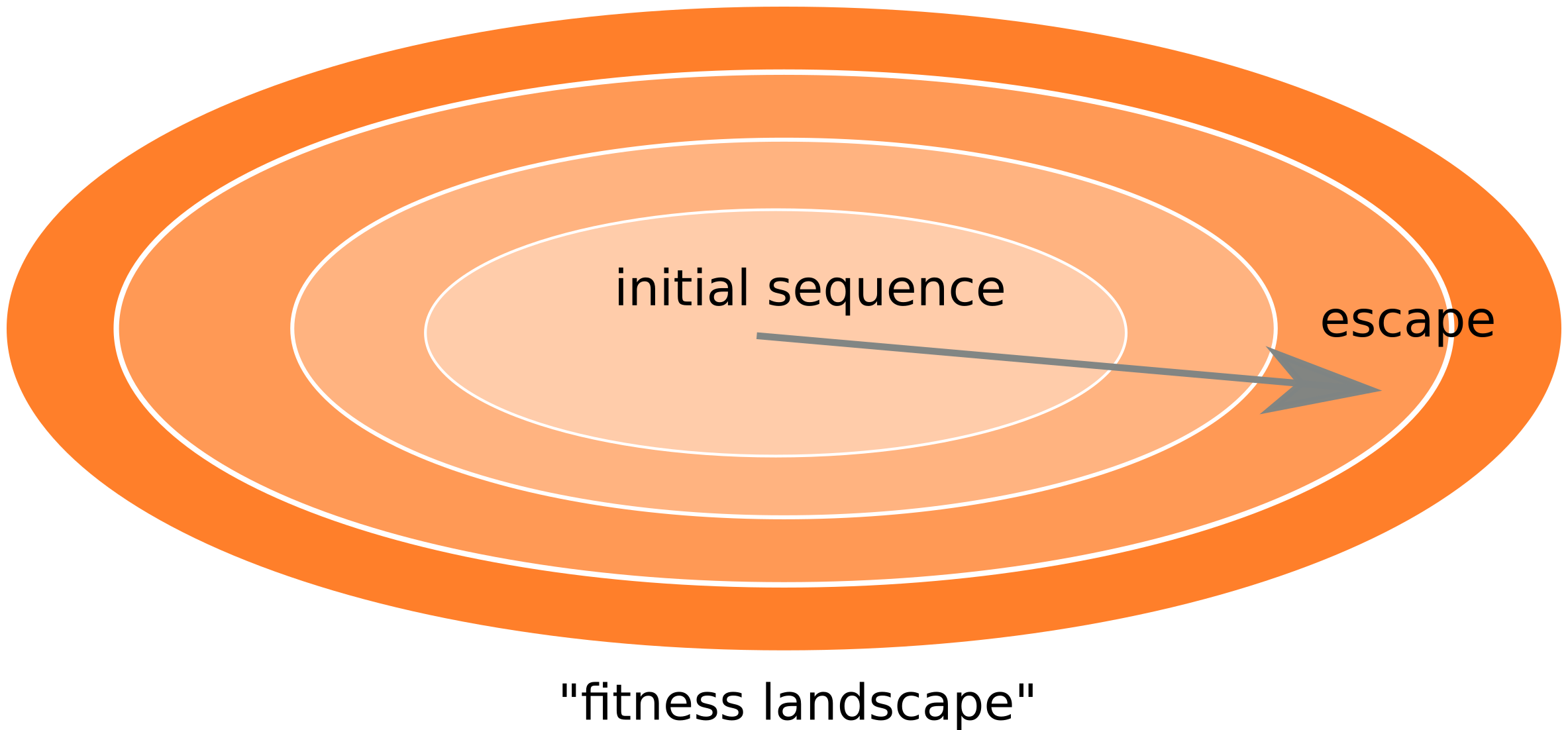
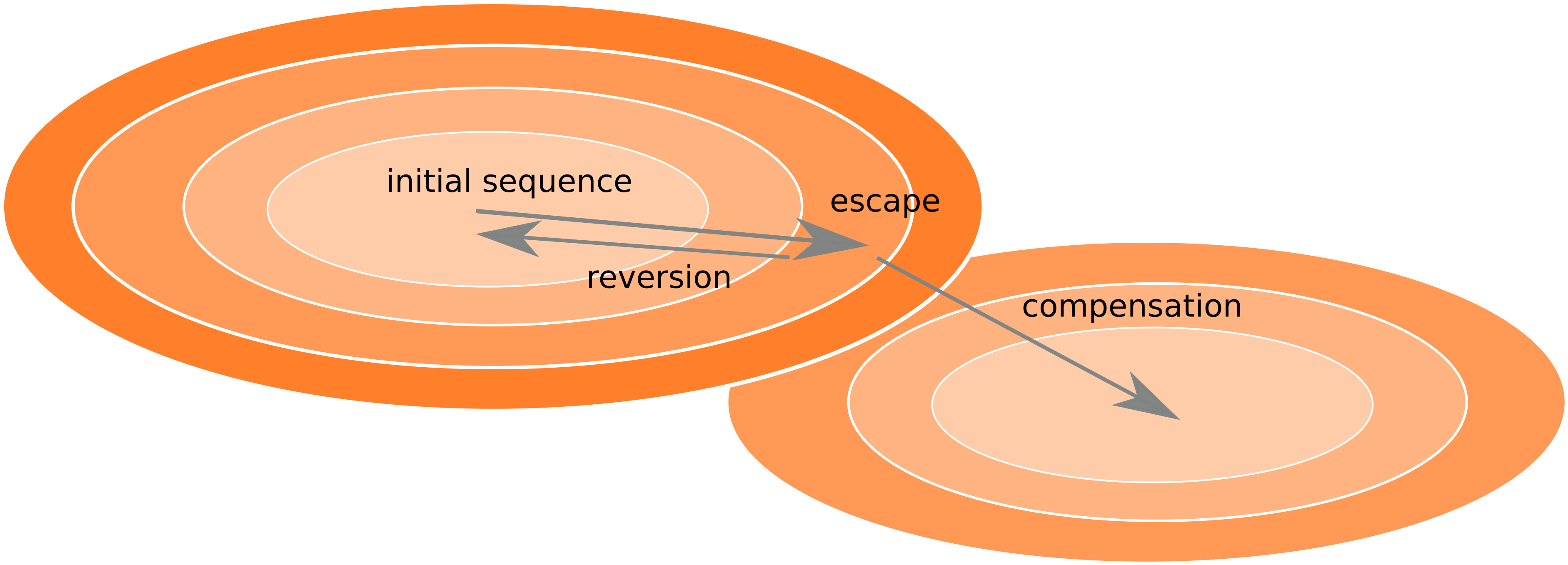
Accurate frequency estimates by averaging many samples
- Frequencies of costly mutations decorrelate fast $\frac{d x}{dt} = \mu -s x $
- $\Rightarrow$ average many samples to obtain accurate estimates