Reconstructing, tracking, and predicting viral spread and evolution
Richard Neher
Biozentrum & SIB, University of Basel
slides at neherlab.org/202205_Cologne.html
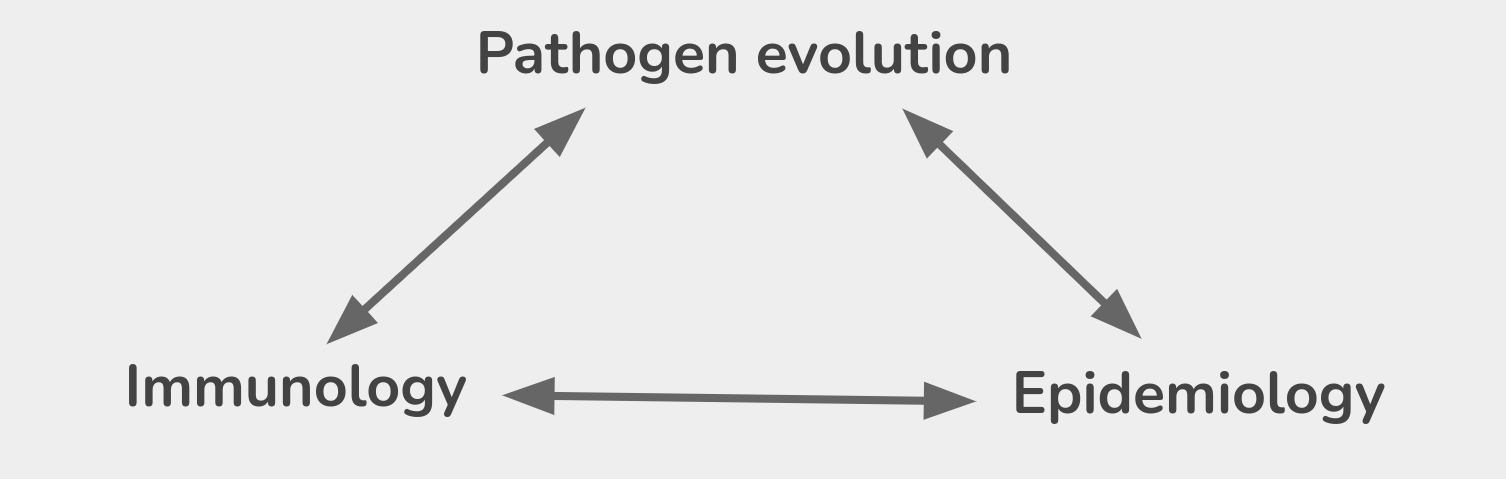
Genomic analysis to reconstruct pathogen spread and evolution
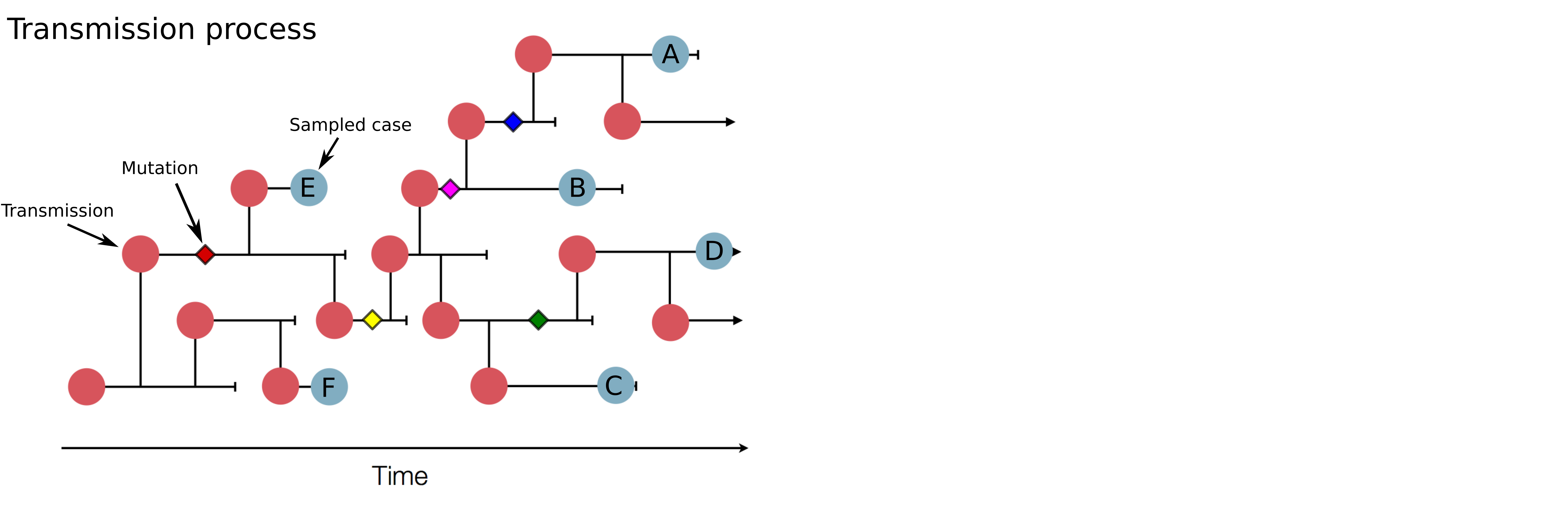
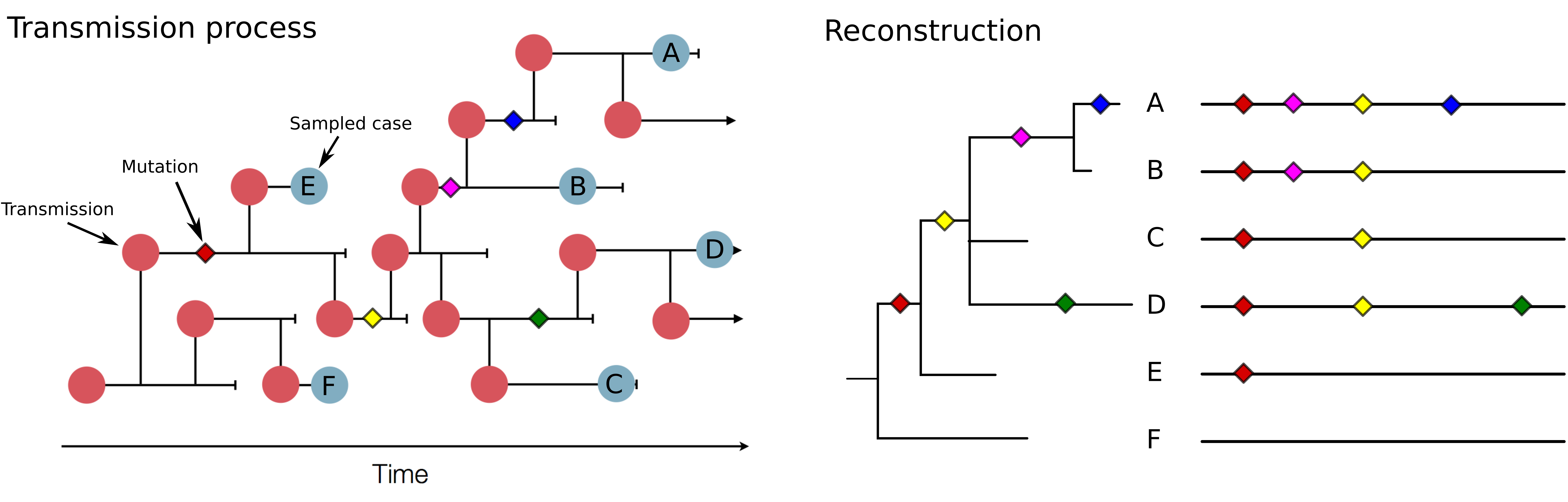
Track how pathogens spread: clusters, introductions, etc
Link genotypic and phenotypic changes: immune escape, drug resistance, host adaptation
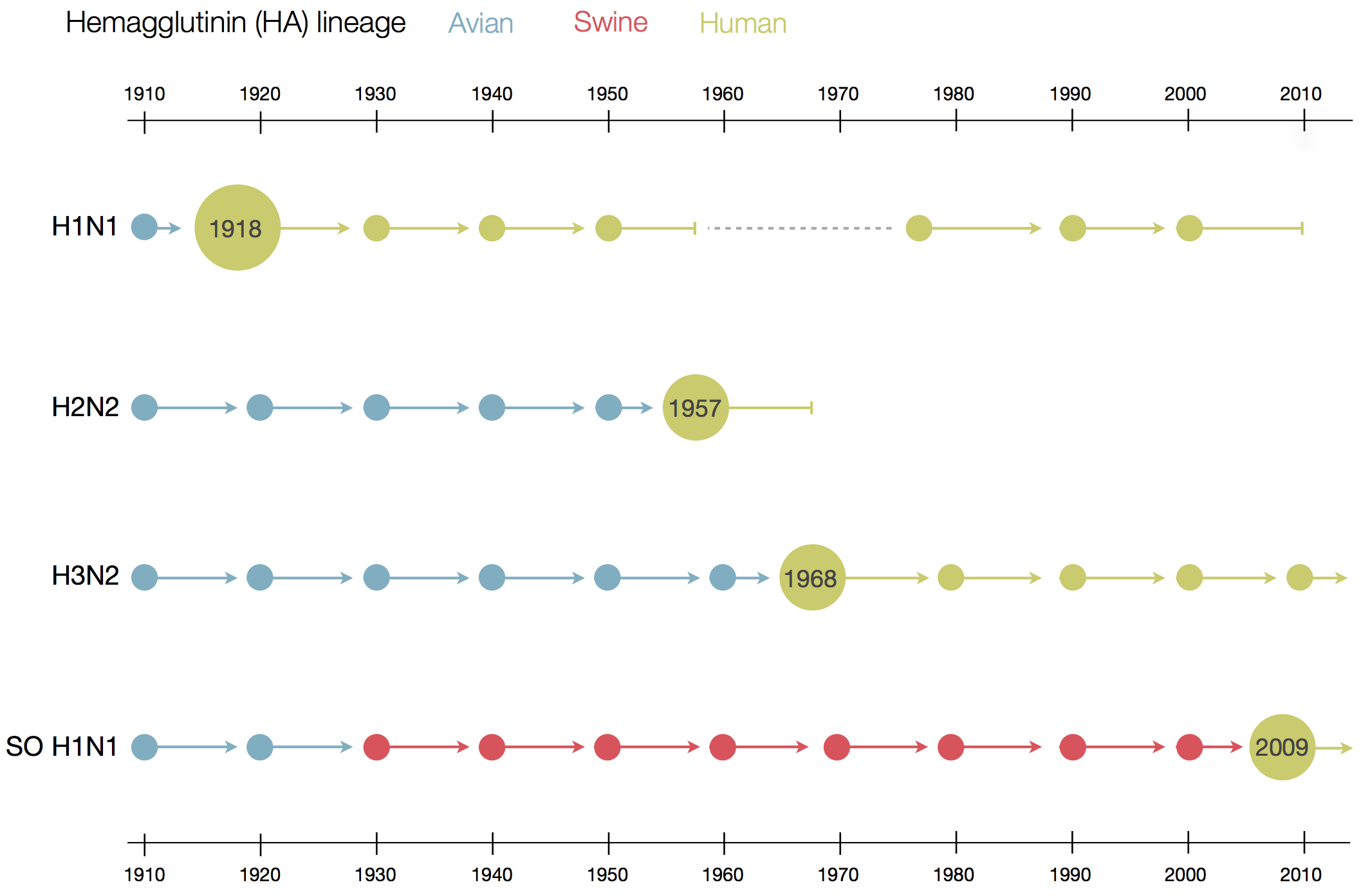
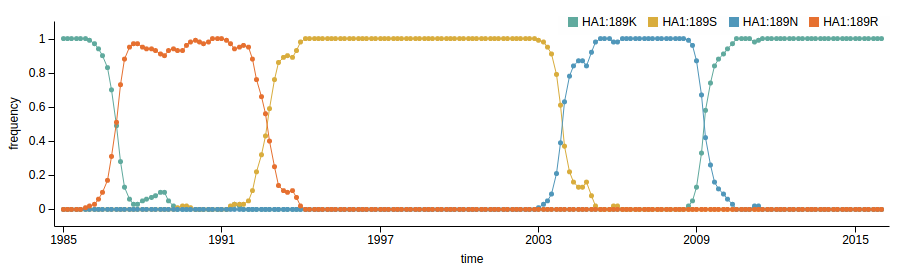
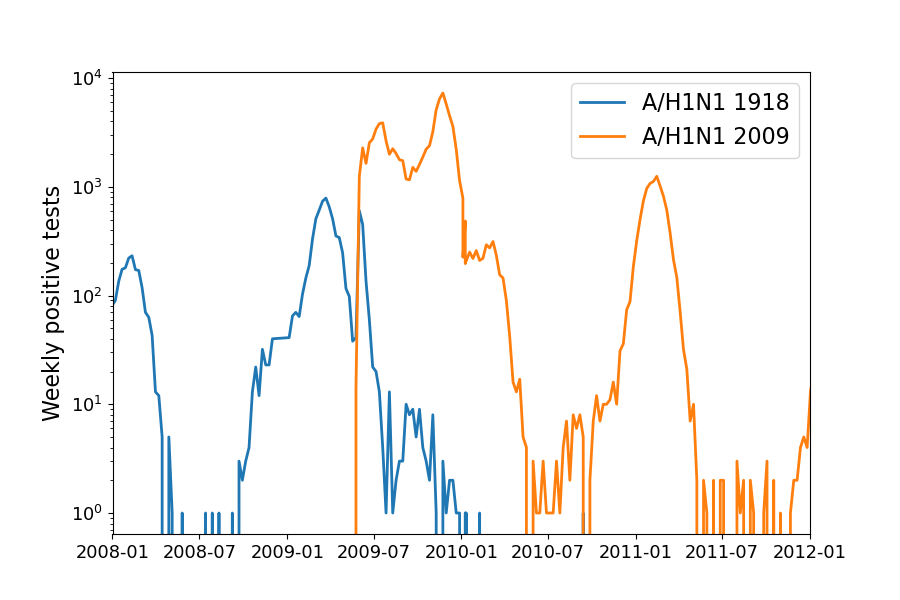
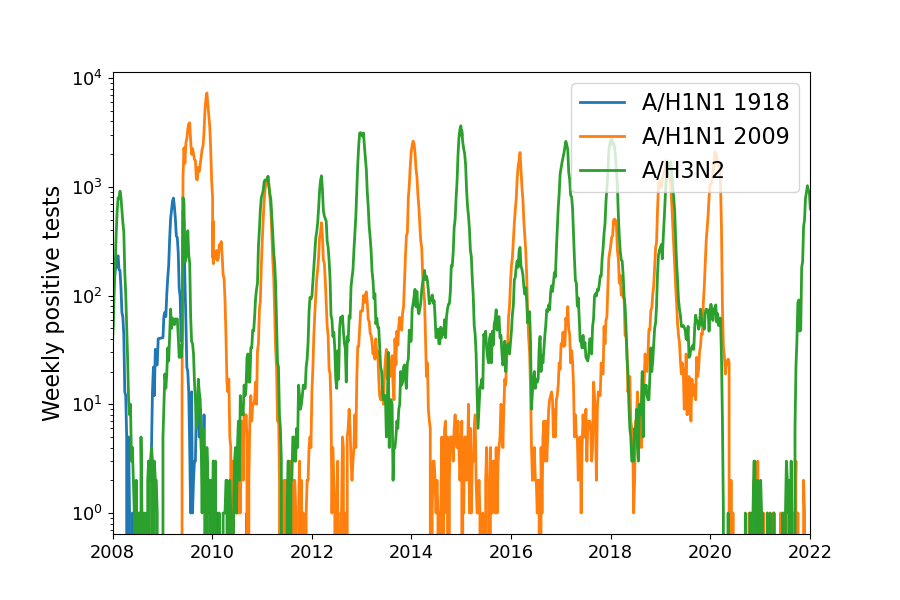
Human seasonal influenza viruses
Positive tests for influenza in the USA by week
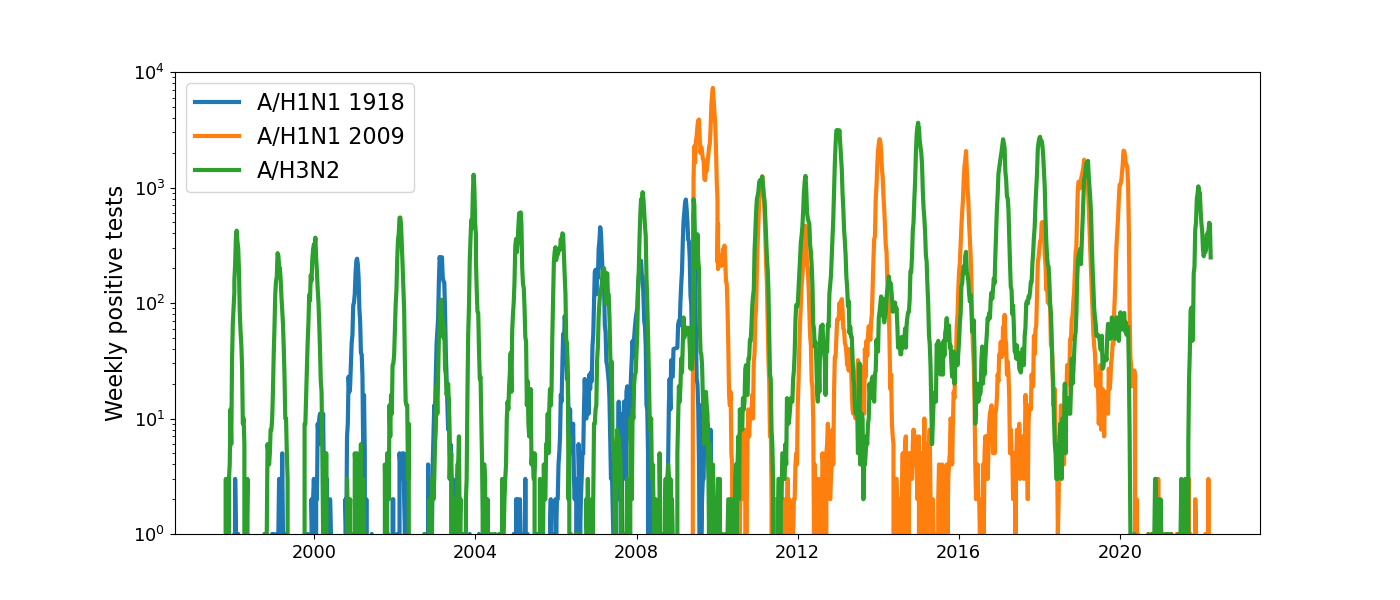 Data by the US CDC
Data by the US CDC
Continuous circulation requires steady "supply" of susceptibles
- Birth of immunologically naive individuals
- Waning of pre-existing immunity
- Antigenic change of the virus
Measles (pre vaccine)
- extremely rapid infection of naive children
- long-lived immunity → rare re-infection
Influenza
- frequent reinfection of adults
- combination of waning and antigenic evolution
- Approximate equilibrium between immunity and infections
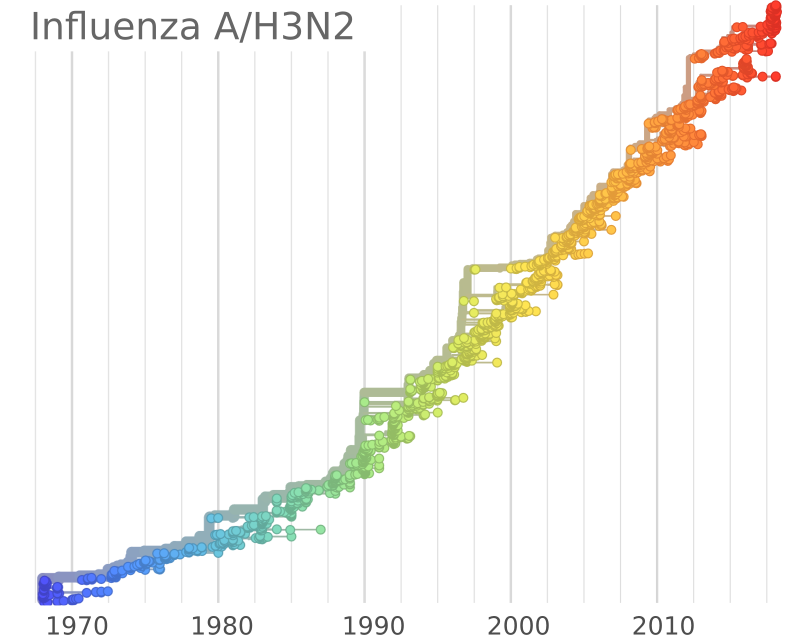
Influenza A/H3N2
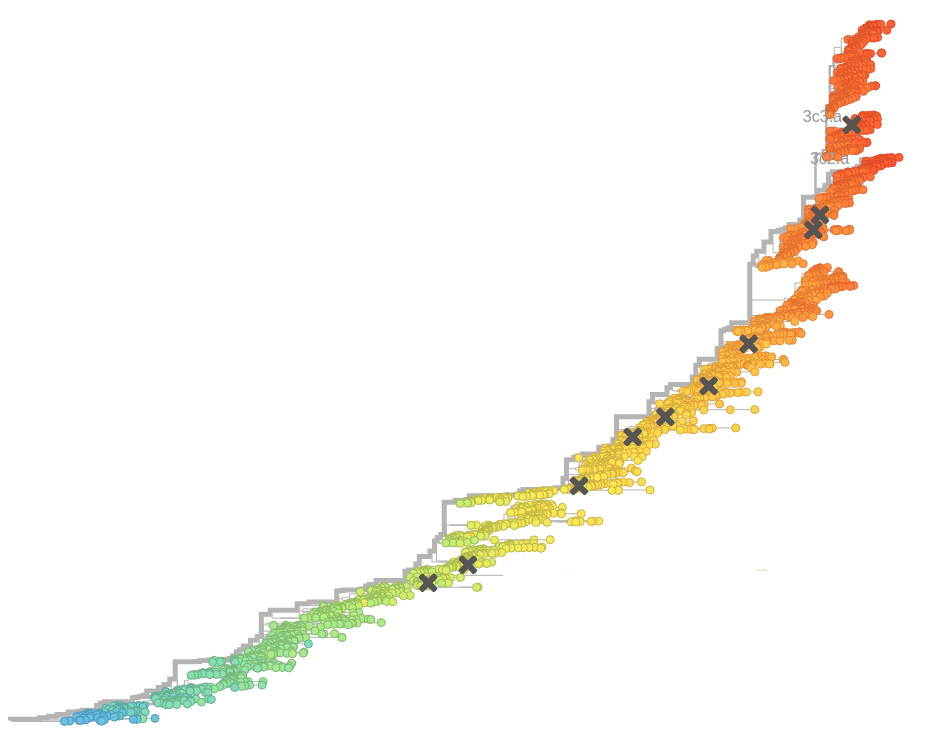
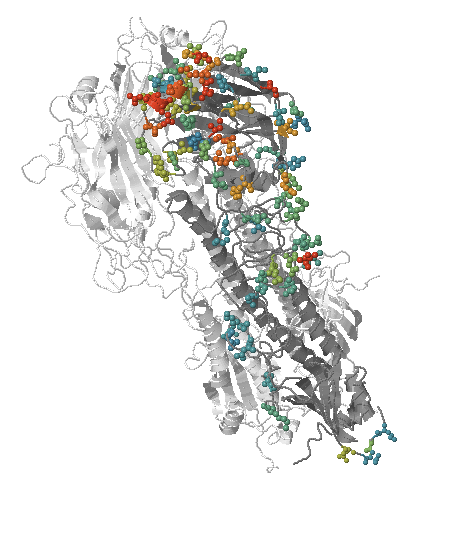
- Influenza viruses evolve to avoid human immunity
- Vaccines need frequent updates
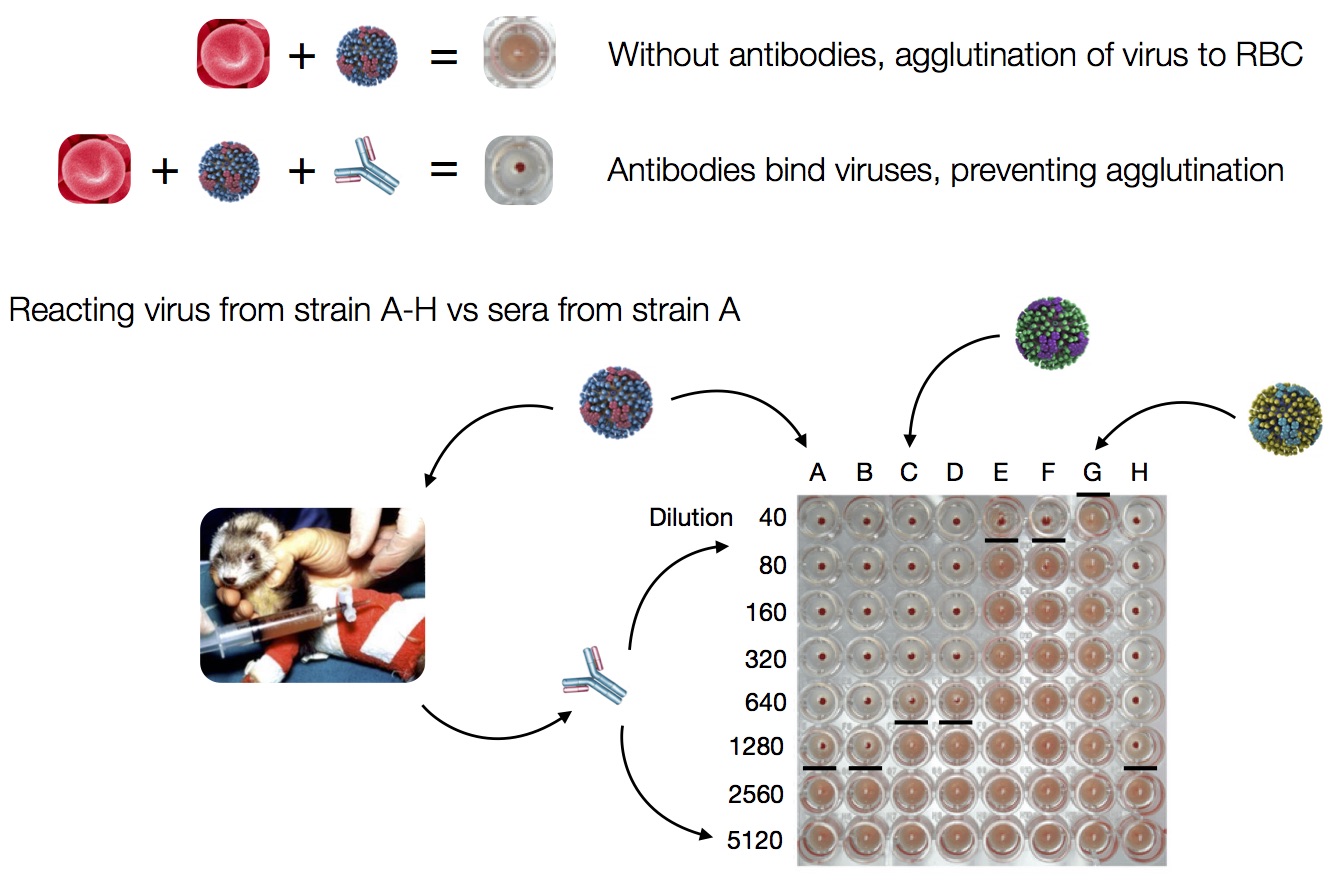
Hemagglutination Inhibition assays
Antigenic distance tables
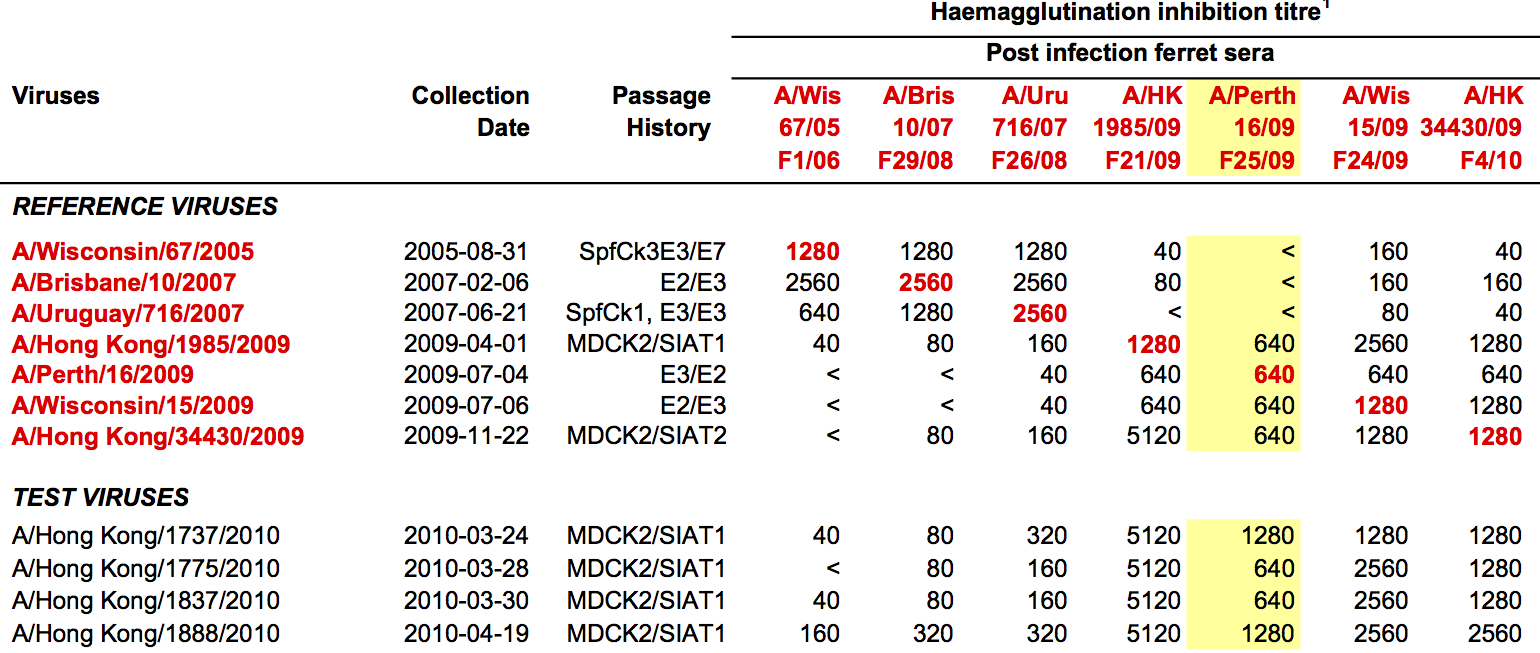
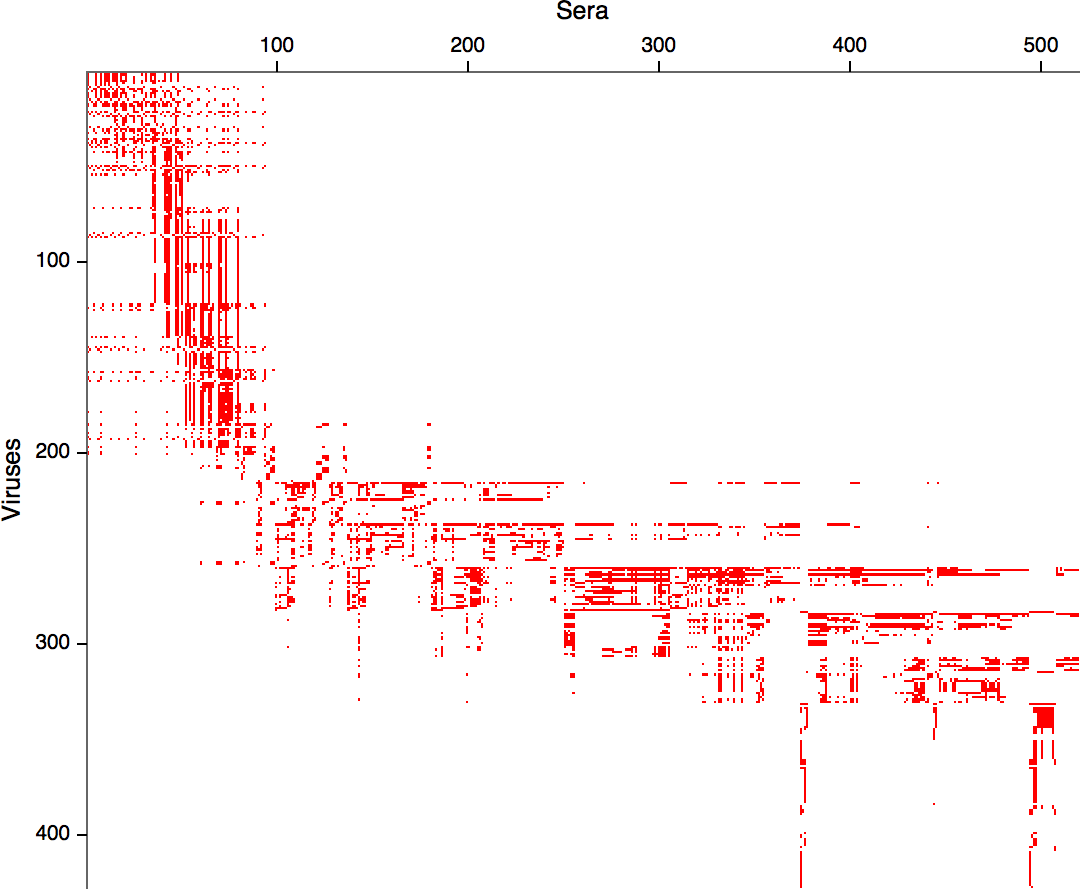
- Long list of distances between sera and viruses
- Tables are sparse, only close by pairs
Integrating antigenic and molecular evolution
- each branch contributes $d_i$ to antigenic distance
- sparse solution for $d_i$ through $l_1$ regularization
Integrating antigenic and molecular evolution -- ferret serology
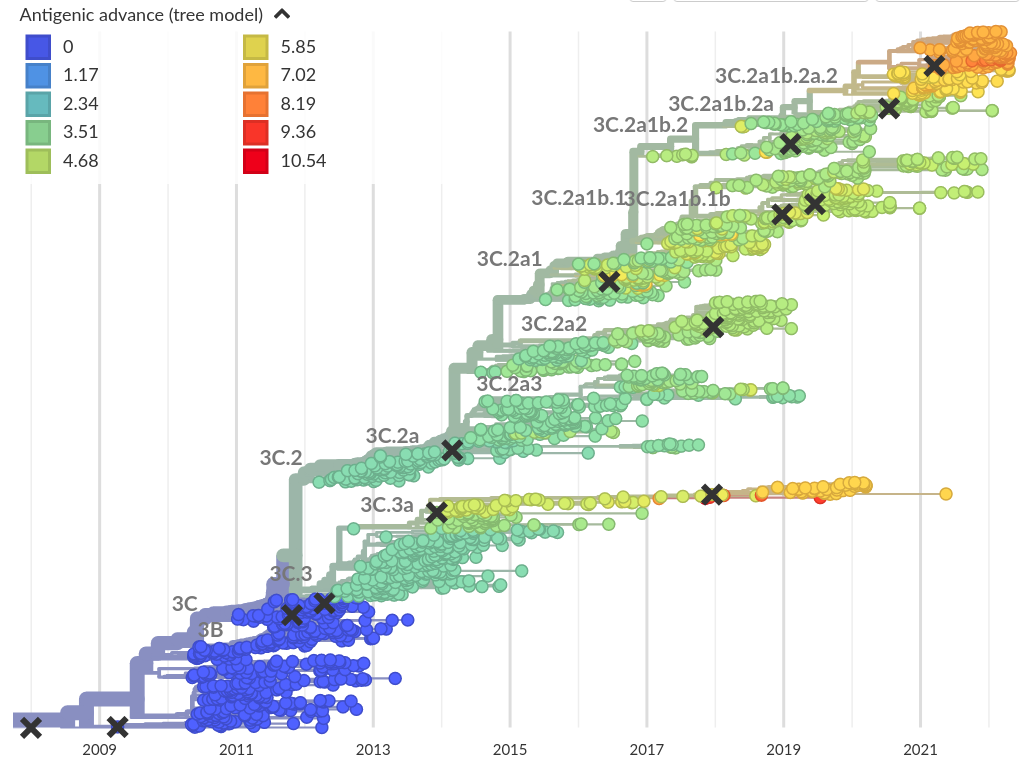 RN et al, PNAS, 2017
RN et al, PNAS, 2017
nextflu.org
joint project with Trevor Bedford & his lab
Transition from pandemic to endemic: 2009 A/H1N1
Simple SIR models predict over-shoot and extinction
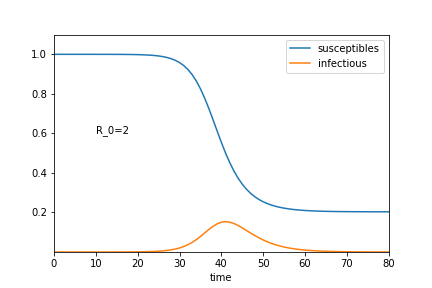 Final susceptibility $S_\infty$: $\log(S_\infty) = - R_0 (S_\infty-1)$
Final susceptibility $S_\infty$: $\log(S_\infty) = - R_0 (S_\infty-1)$ $R_0=2$ → $S_\infty = 0.2$, very small for later $R_0$.
Positive tests for influenza during the 2009 pandemic
 Data by the US CDC
Data by the US CDC
Positive tests for influenza during the 2009 pandemic
 Data by the US CDC
Data by the US CDC
Antigenic evolution during the pandemic-to-endemic transition
Model with strains $a,b,c,\ldots$:
Infections: $ \frac{d}{dt}I_a = \beta S_a I_a - \nu I_a $
Susceptibilty: $ \frac{d}{dt}S_a = -\nu S_a \sum_{b} K_{ab}I_b $ cross-immunity: $K_{ab}$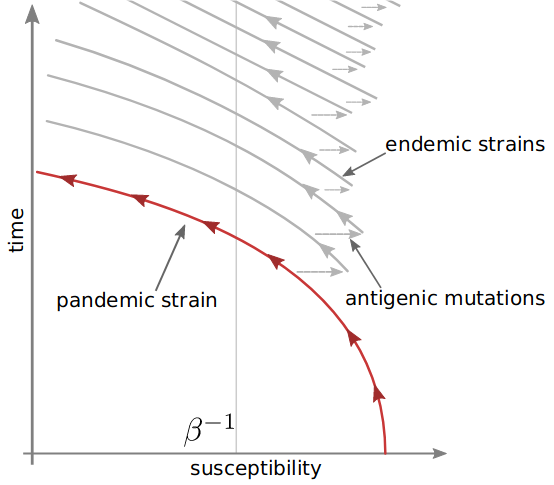
Infections: $ \frac{d}{dt}I_a = \beta S_a I_a - \nu I_a $
Susceptibilty: $ \frac{d}{dt}S_a = -\nu S_a \sum_{b} K_{ab}I_b $ cross-immunity: $K_{ab}$

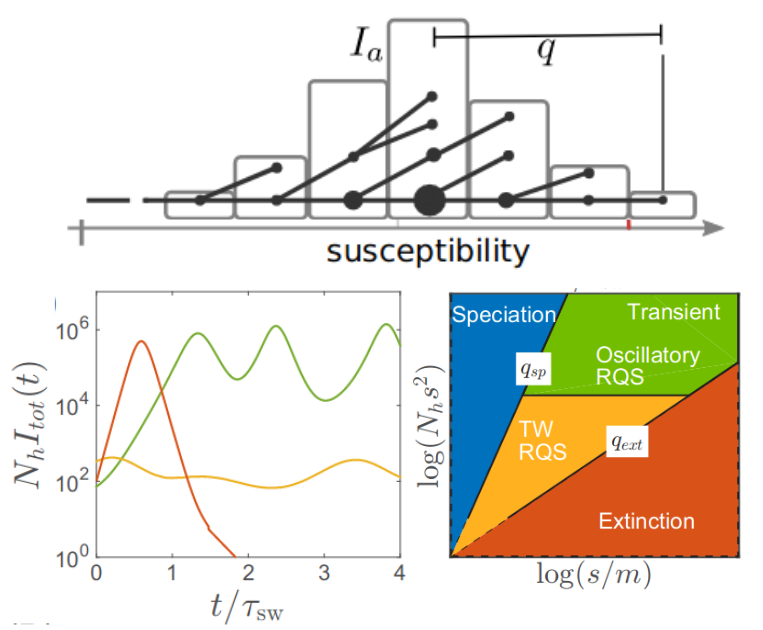
- early large antigenic steps (relative to cross-immunity range) are necessary to persist
- large cross-immunity range is necessary to prevent speciation
- cross-immunity typically has short and long range components
Speciation?
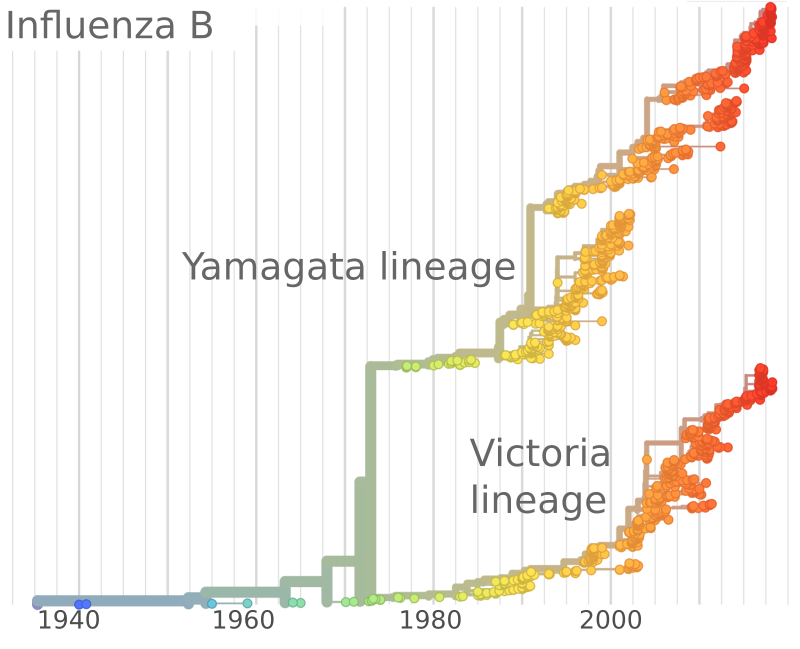
- The Yamagata lineage might have disappeared during the pandemic
SARS-CoV-2
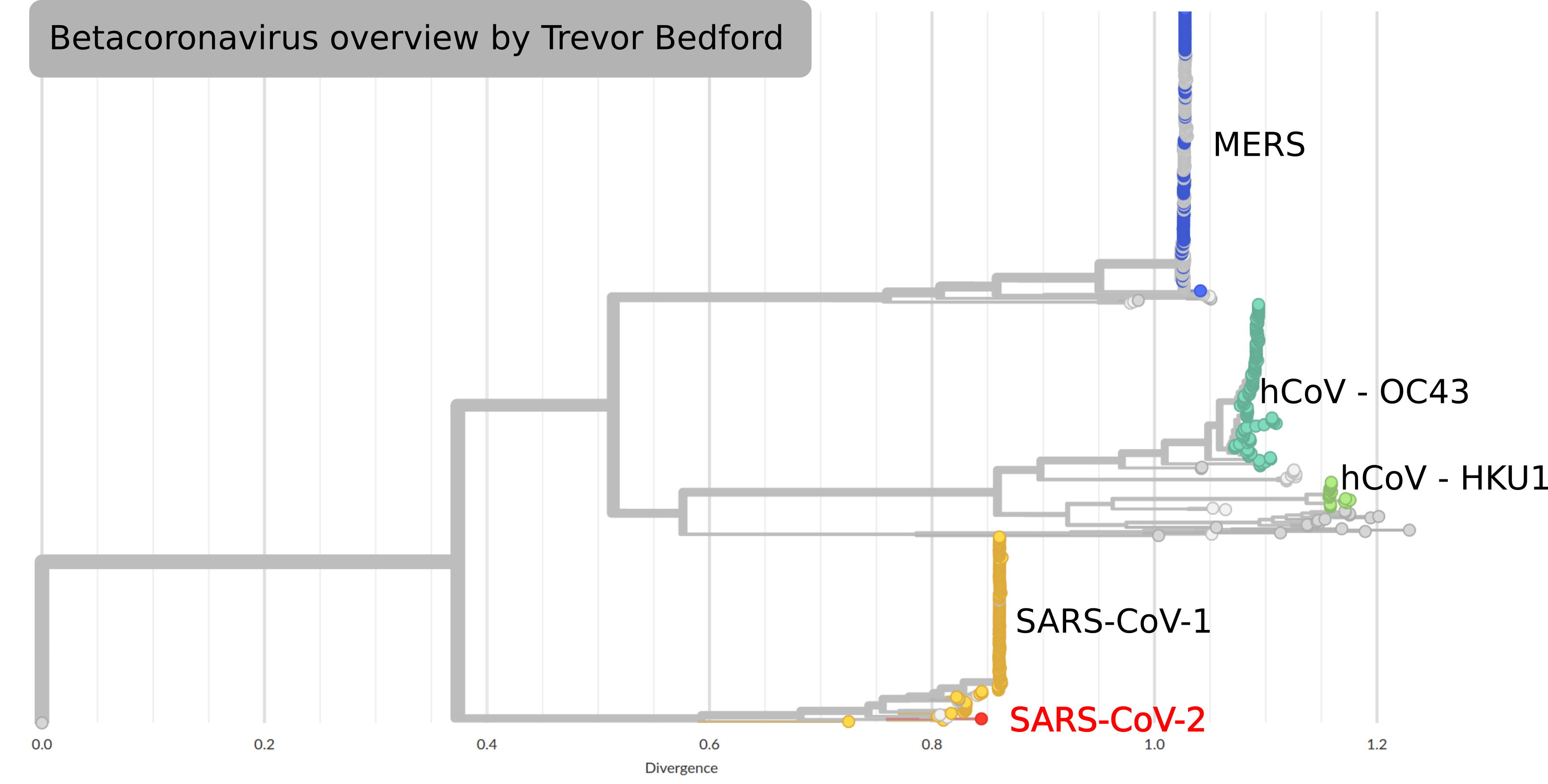 by Trevor Bedford
by Trevor Bedford
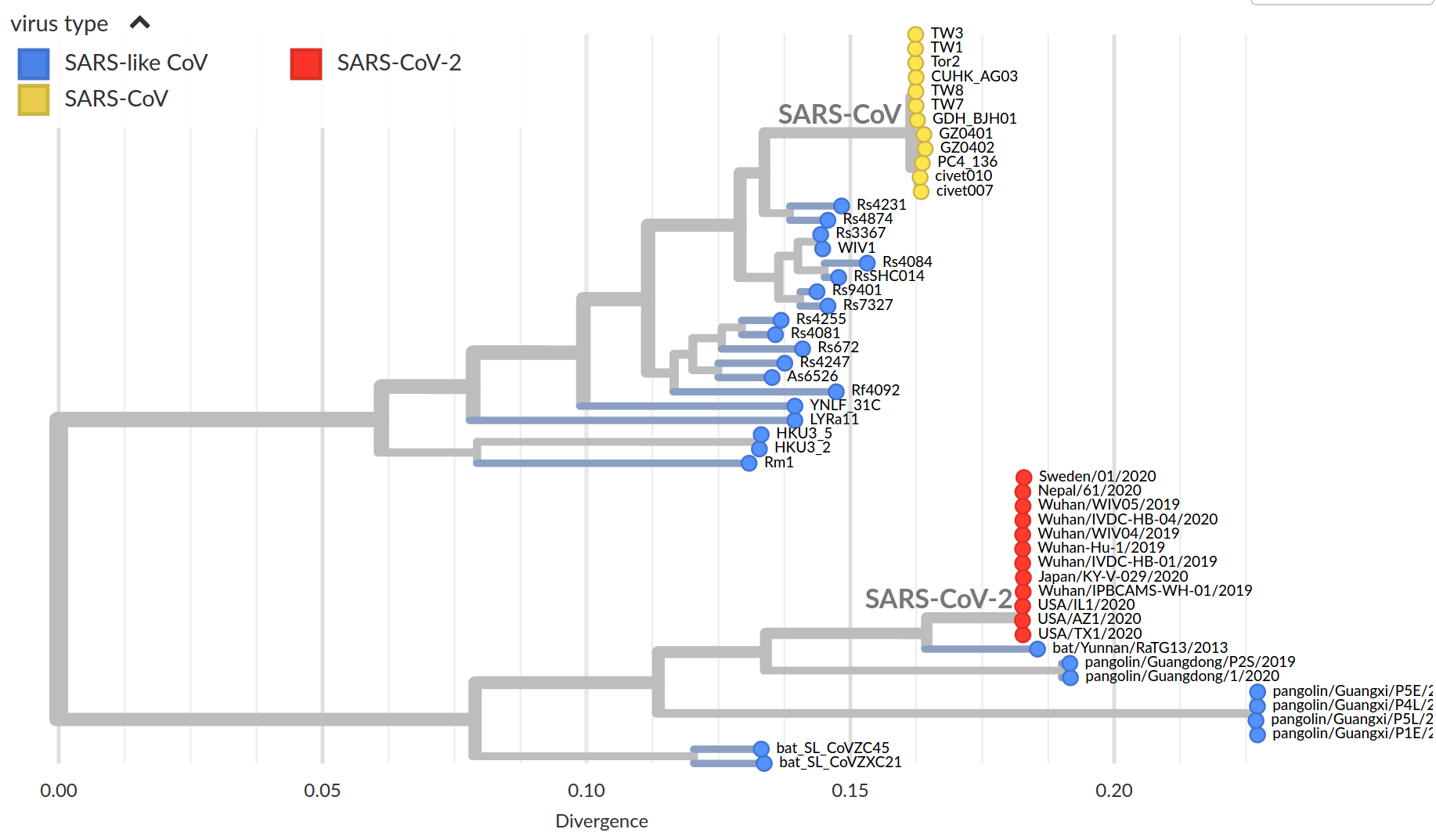 by Trevor Bedford
by Trevor Bedford
Available data on Jan 26 2020
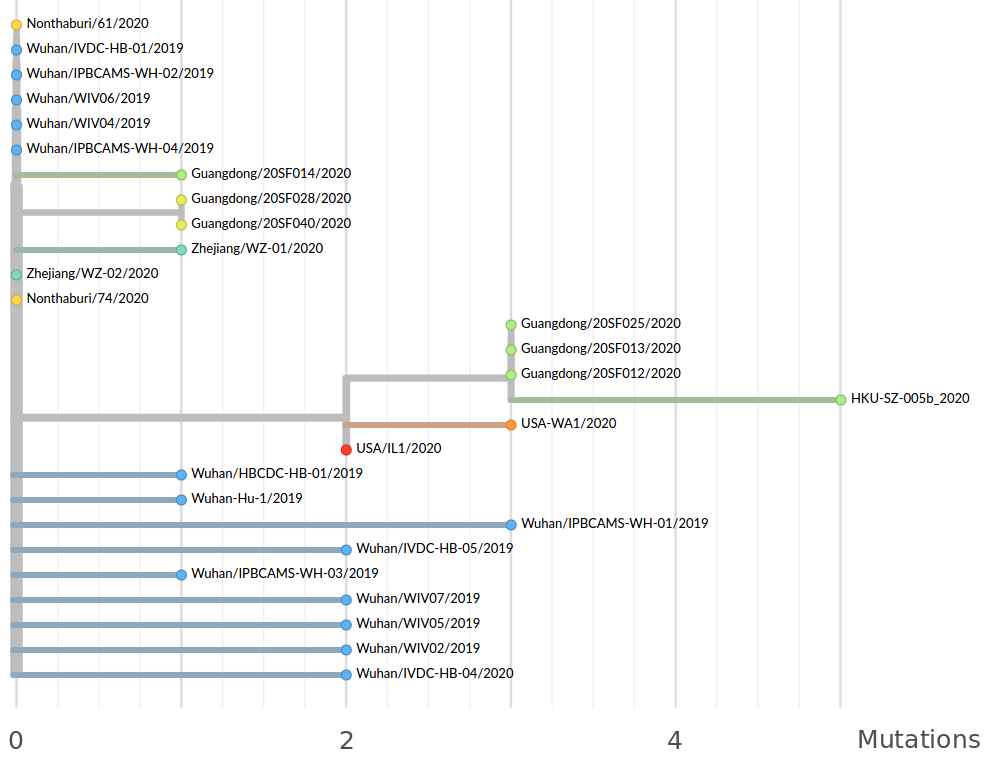
Early genomes differed by only a few mutations, suggesting very recent emergence
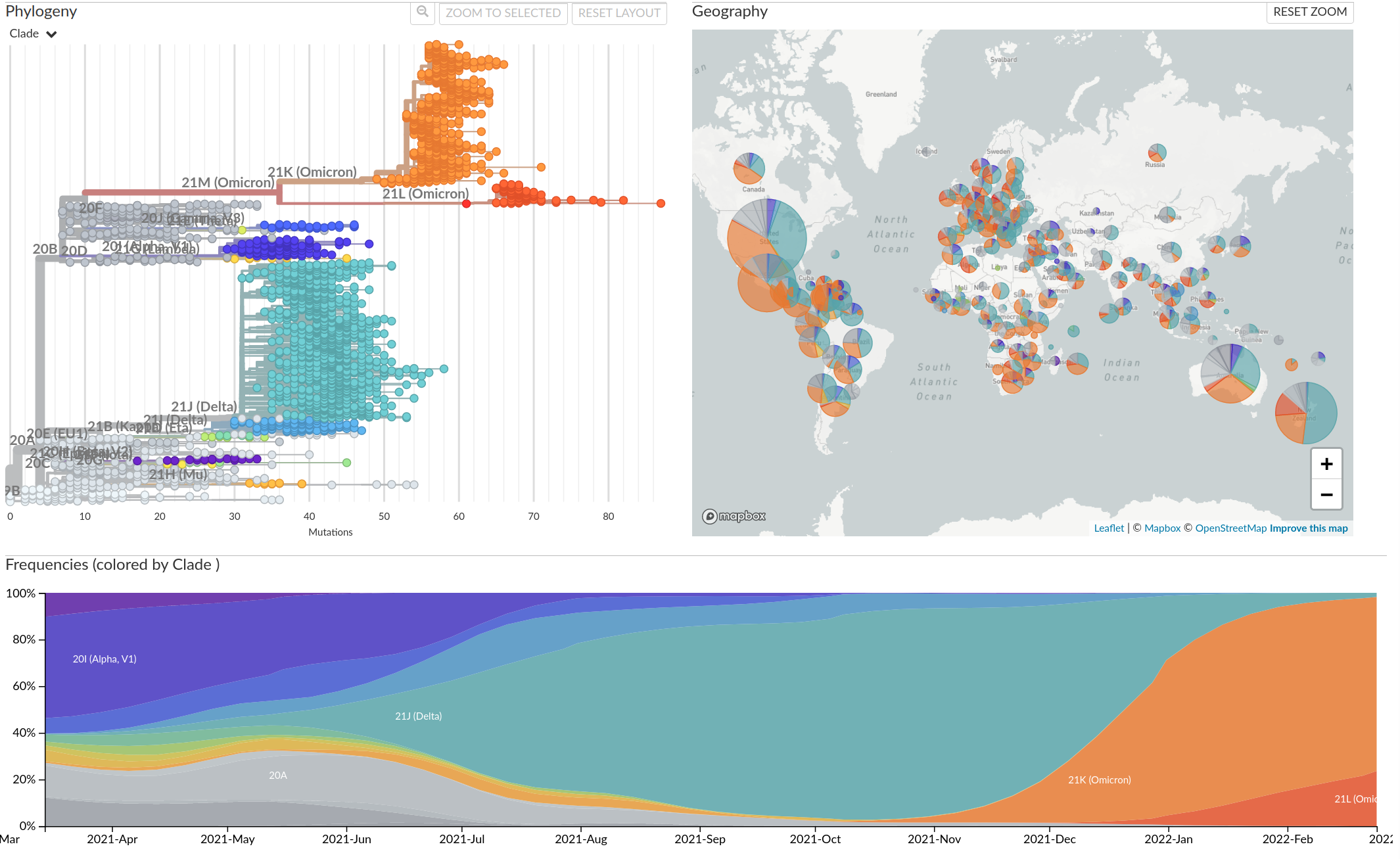
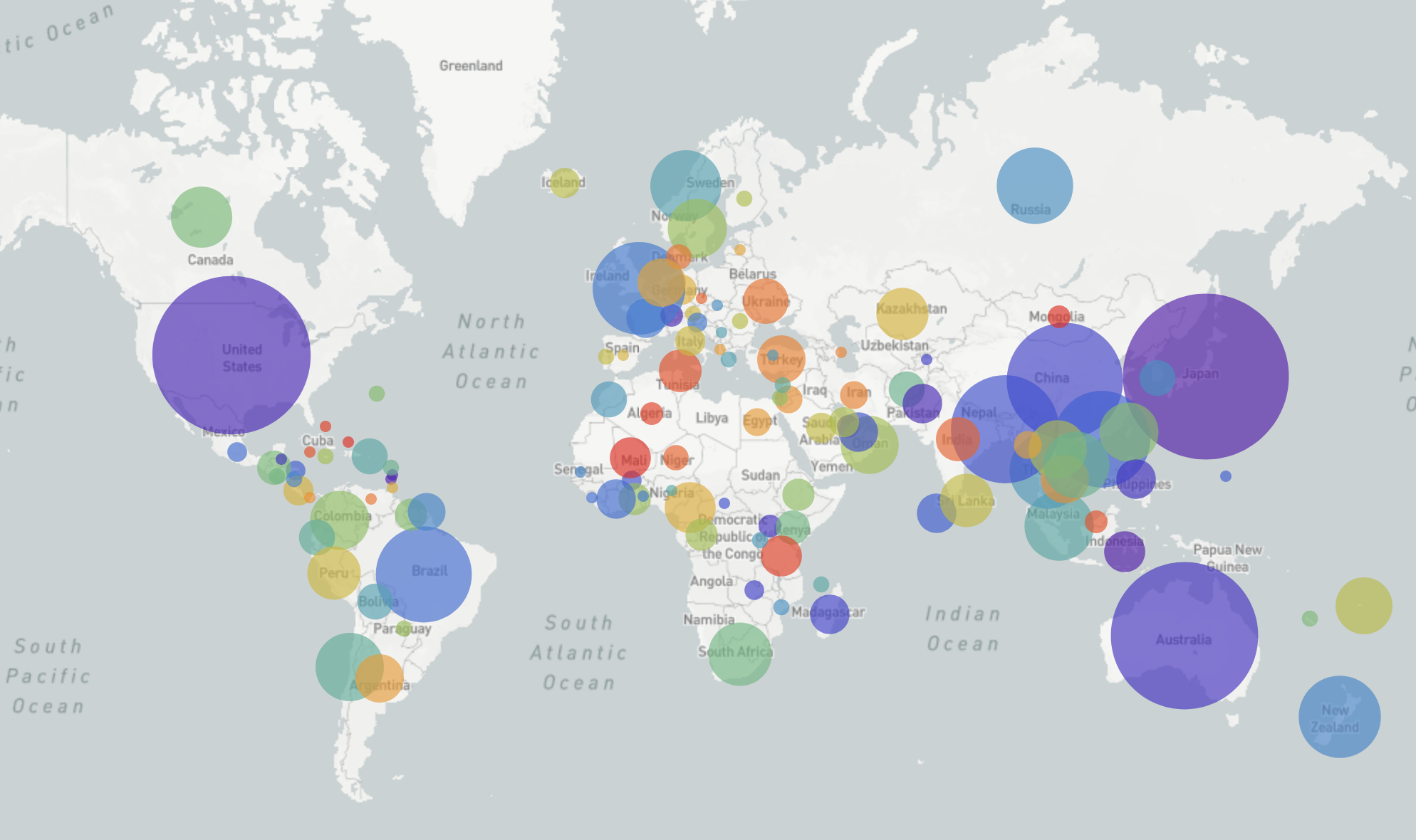
Tracking diversity and spread of SARS-CoV-2 in Nextstrain
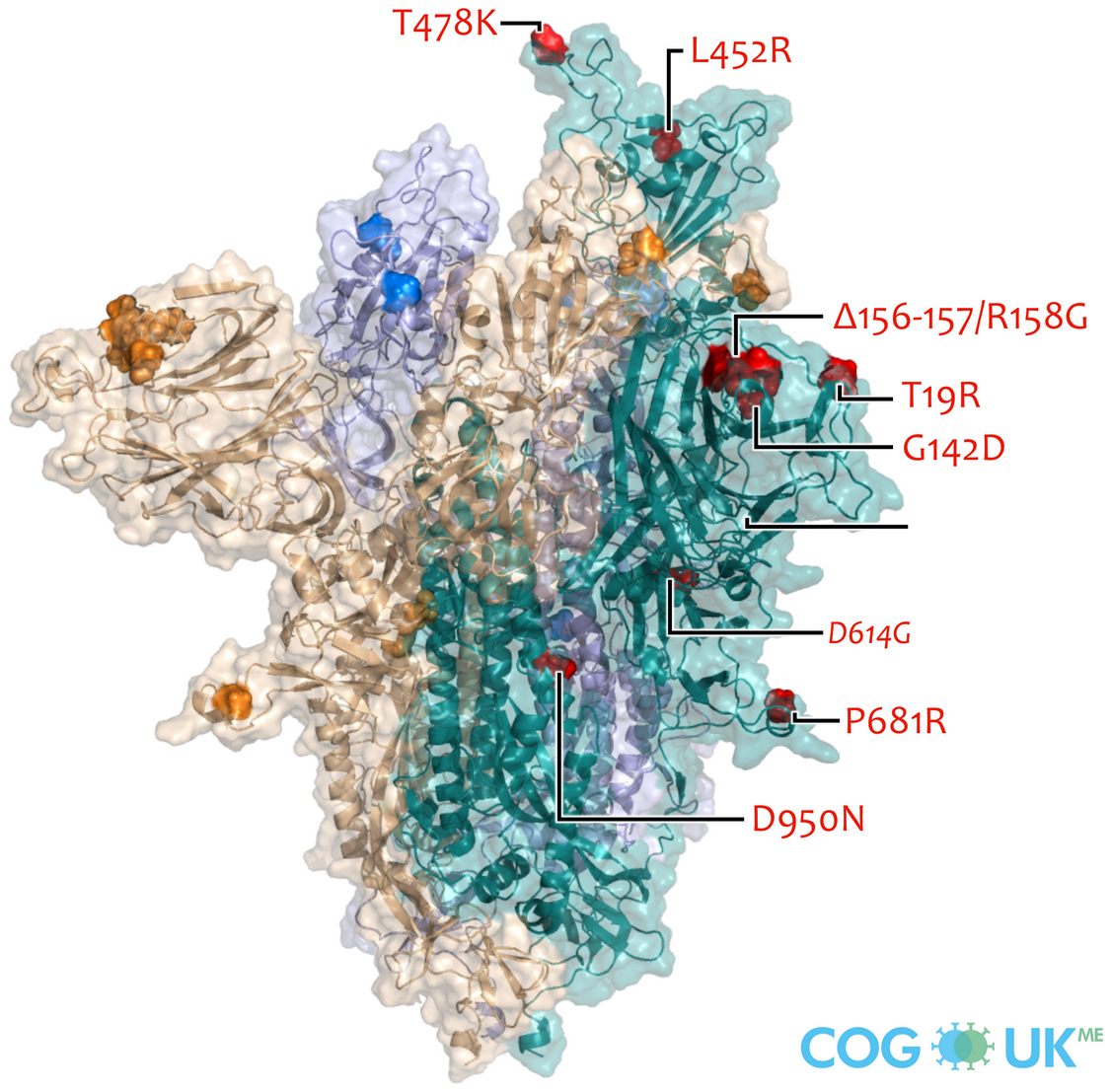
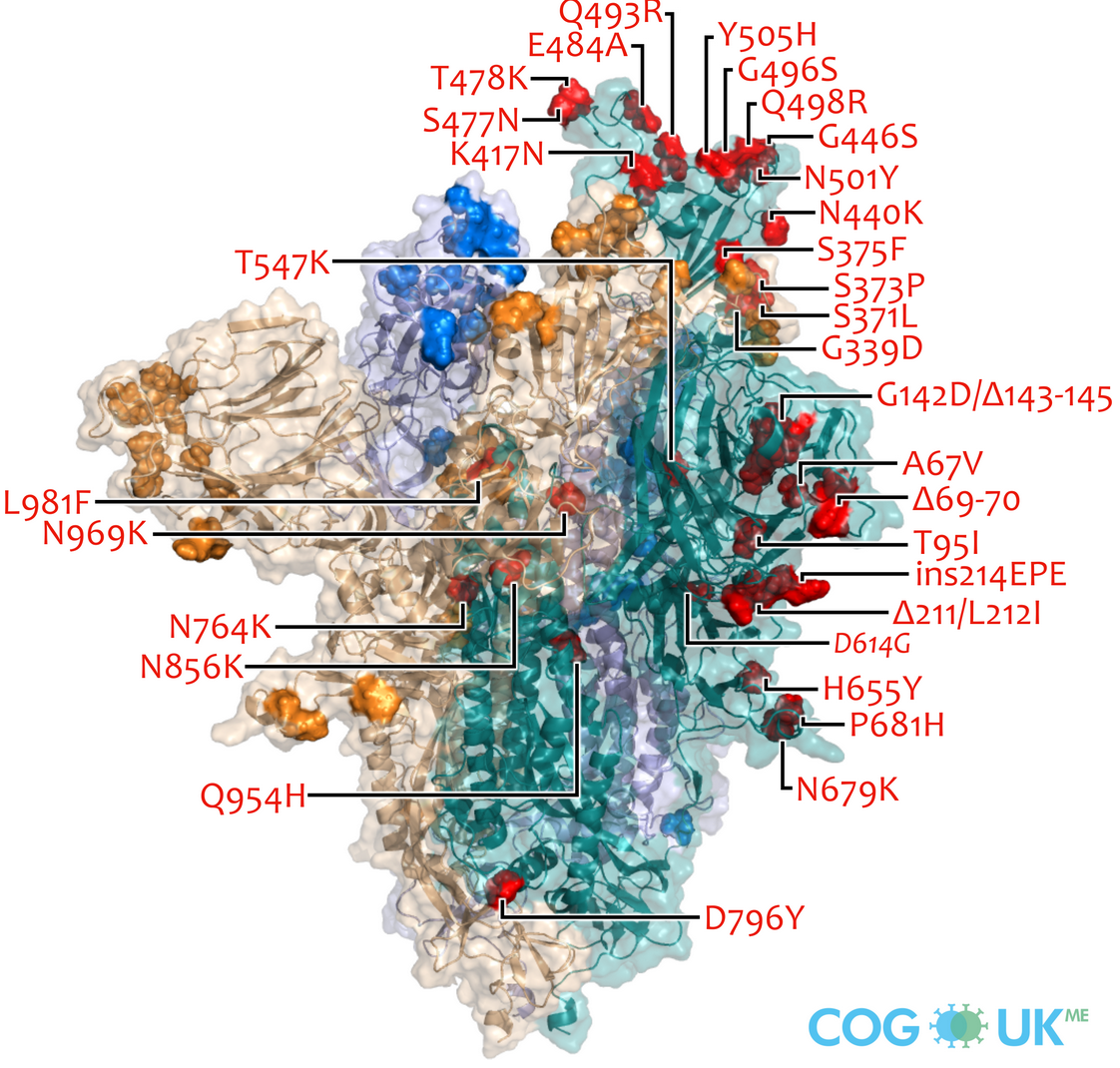
Successful variants are characterized by many mutations in S1
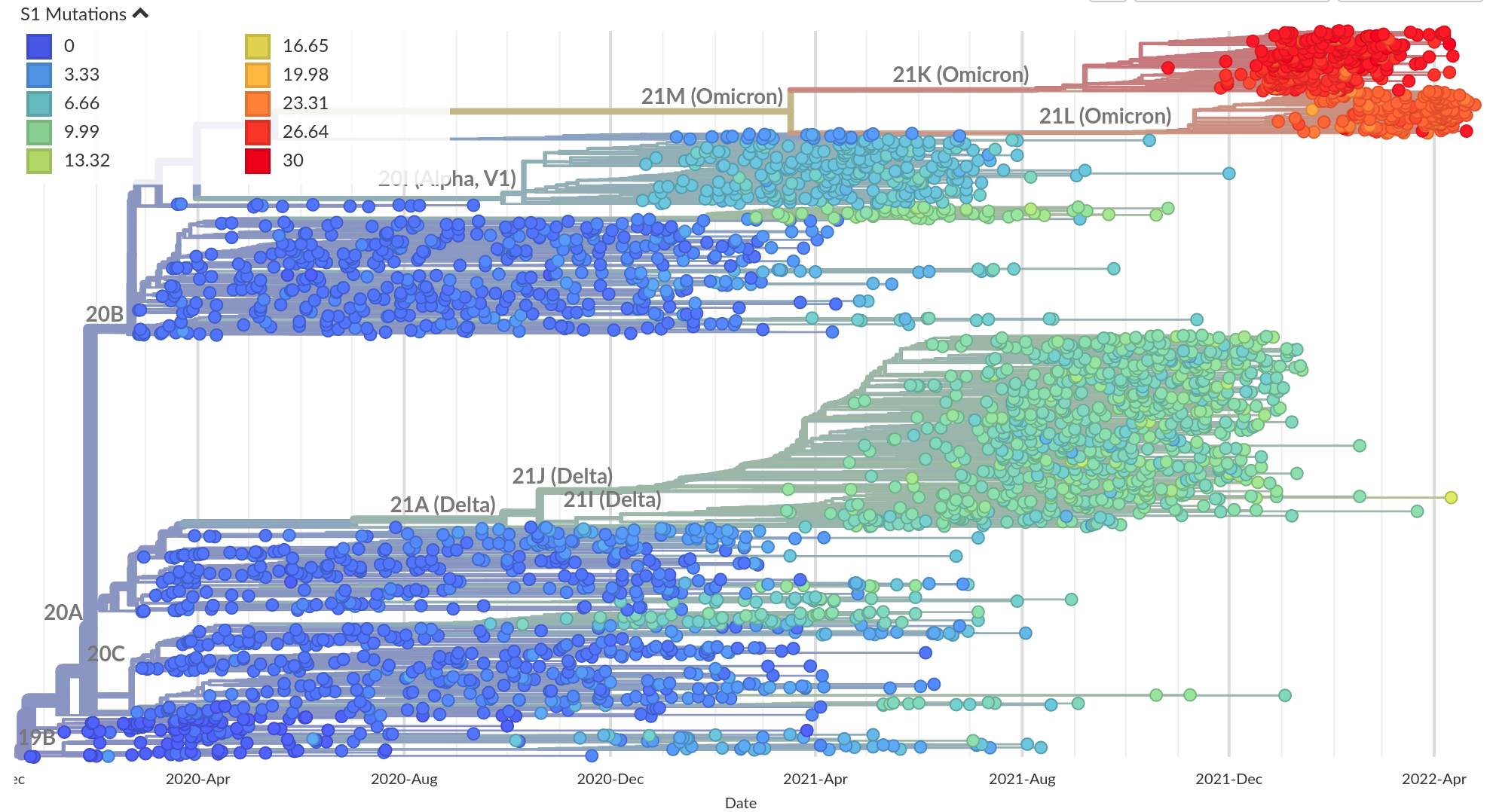
Successful variants are characterized by many mutations in S1
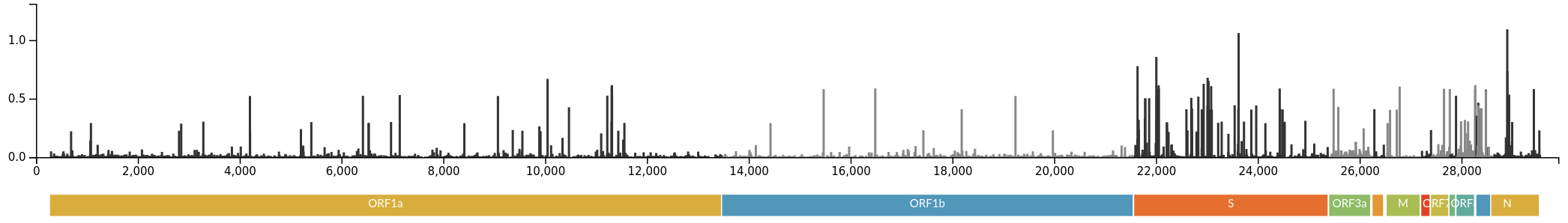
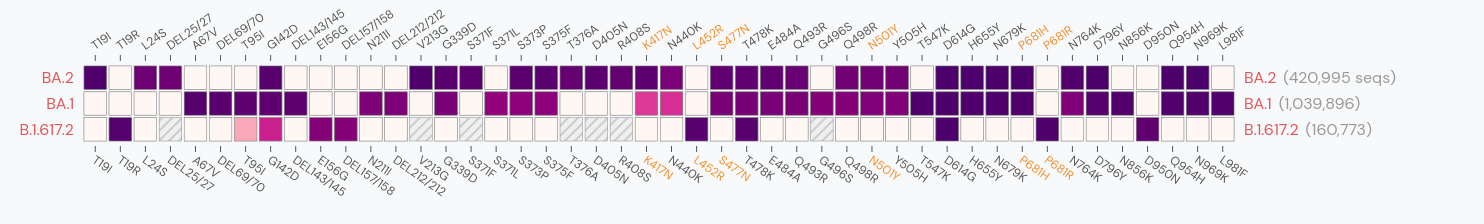
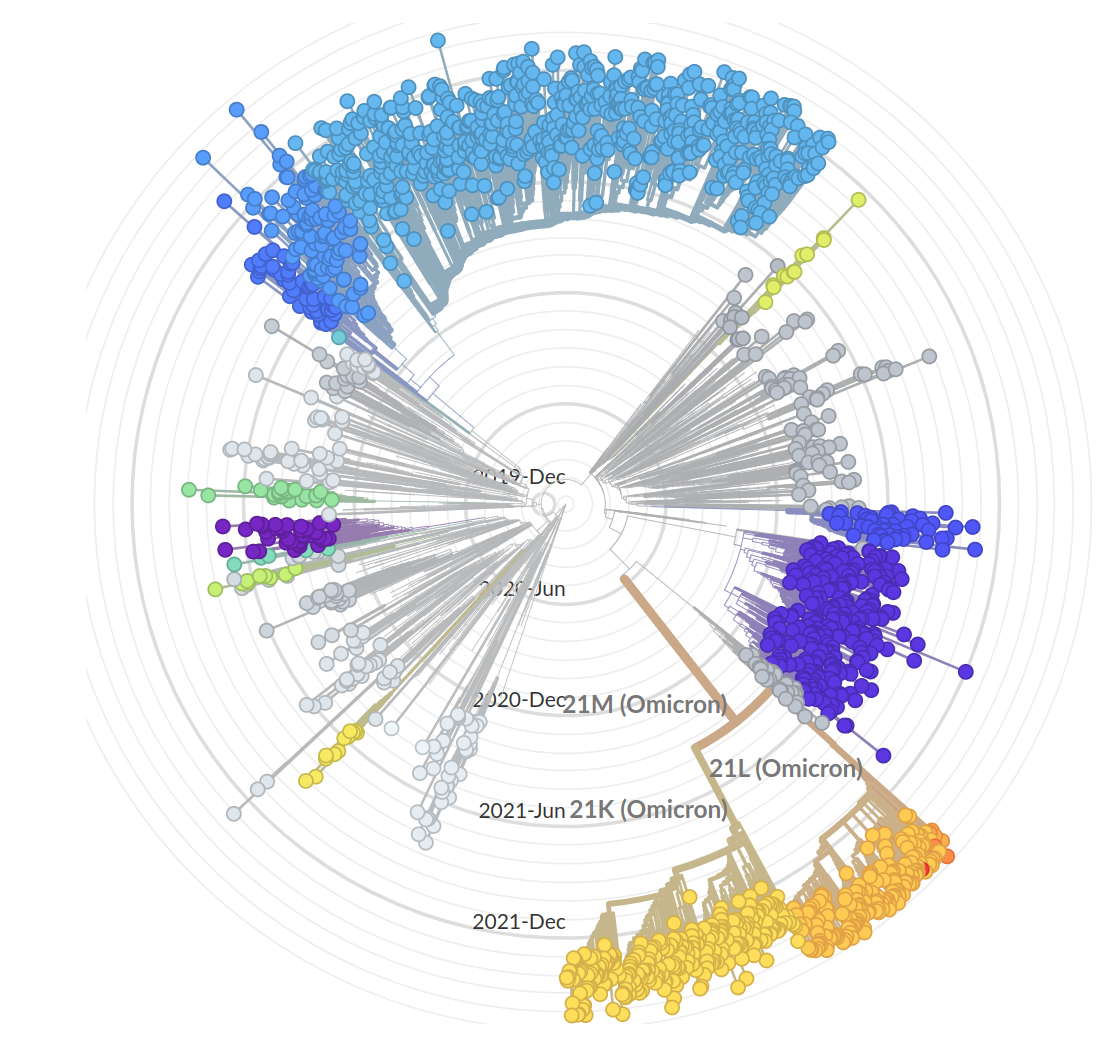
Delta vs Omicron
So far, independent variants have dominated sequentially
Current Omicron subvariants of interest
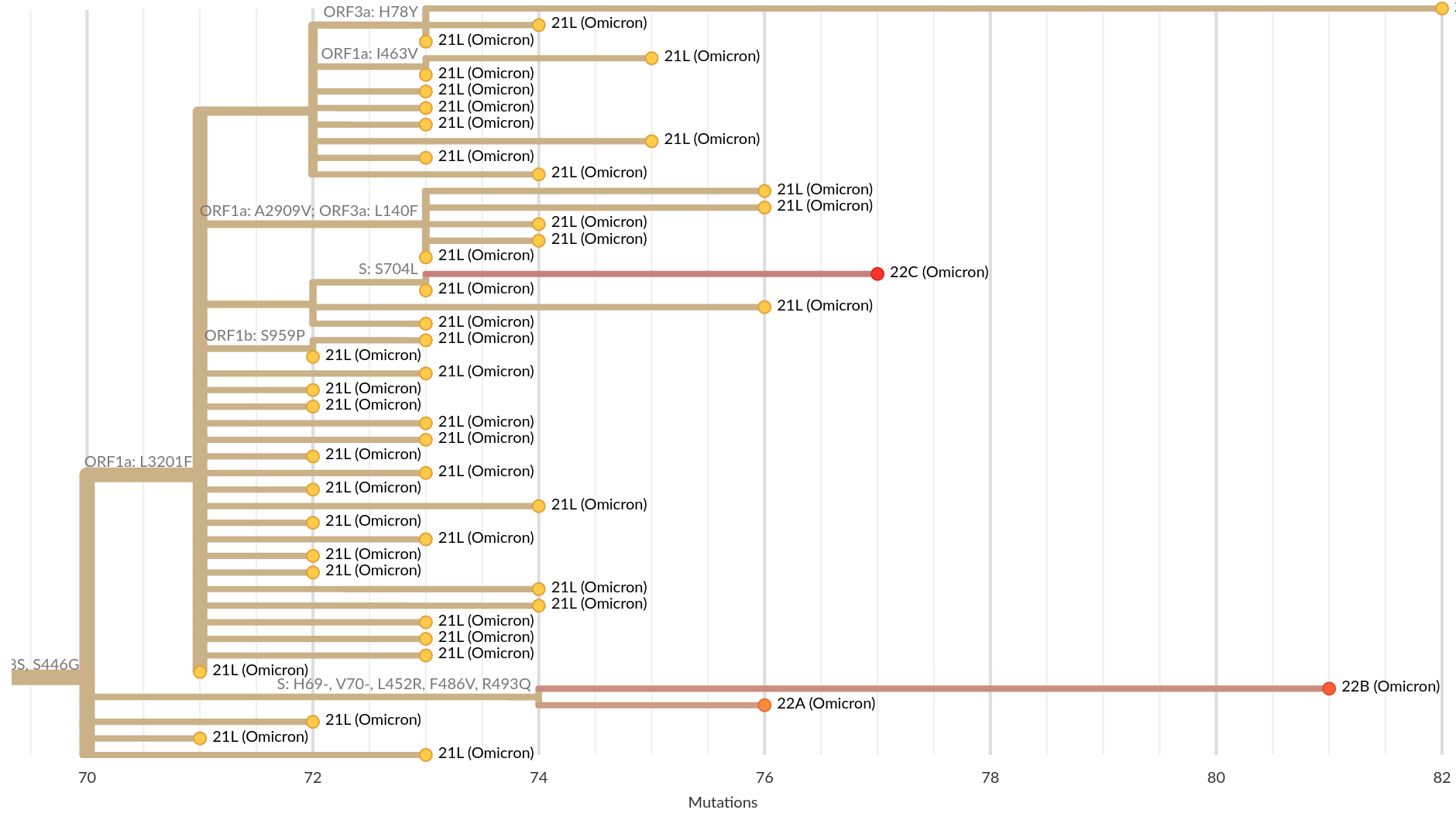
- BA.2 (21L) has essentially taken over.
- BA.4 (22A) and BA.5 (22B) emerged in Southern Africa. Mutations at positions 452 and 486 lead to immune evasion
- BA.2.12.1 (22C) has a mutation at position 452 and is common in the US
Medium term dynamics of SARS-CoV-2 is very uncertain
- Will we start seeing second and third generation variants, as opposed to sister variants?
- Will we the saltatory dynamics with heavily diverged variants continue?
- Will a more diverse immunity landscape slow down future variant dynamics?
- Will waning/antigenic evolution slow down and give rise to annual or even rarer waves?
Influenza and Theory acknowledgments





- Boris Shraiman
- Colin Russell
- Trevor Bedford
- Pierre Barrat
- Oskar Hallatschek
- Le Yan
- All the NICs and WHO CCs that provide influenza sequence data
- The WHO CCs in London and Atlanta for providing titer data

Acknowledgments
Trevor Bedford and his lab -- terrific collaboration since 2014

especially James Hadfield, Emma Hodcroft, Ivan Aksamentov, Cornelius Roemer, Moira Zuber, and John Huddleston
Data we analyze are contributed by scientists from all over the world
Data are shared and curated by GISAID

